Question: Which of the following results in a decrease in the entropy of the system? 1) O2(g),300KO2(g),400K 2) H2O(s),0CH2O(,0C 3) N2(g),25CN2(aq),25C 4) NH3(l),34.5CNH3(g),34.5C 5) 2H2O2(g)2H2O(g)+O2(g)
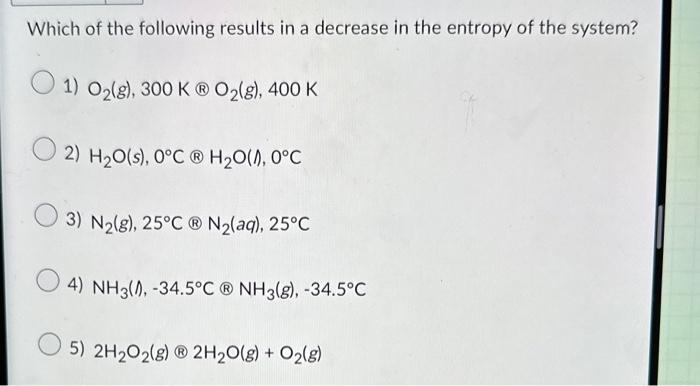
Which of the following results in a decrease in the entropy of the system? 1) O2(g),300KO2(g),400K 2) H2O(s),0CH2O(,0C 3) N2(g),25CN2(aq),25C 4) NH3(l),34.5CNH3(g),34.5C 5) 2H2O2(g)2H2O(g)+O2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


