Question: Which solute has the largest van't Hoff factor (i)? CH4NaOHNa2CO3HCl Question 4 Addition of solute to a solvent causes the solvent vapor pressure to Increase
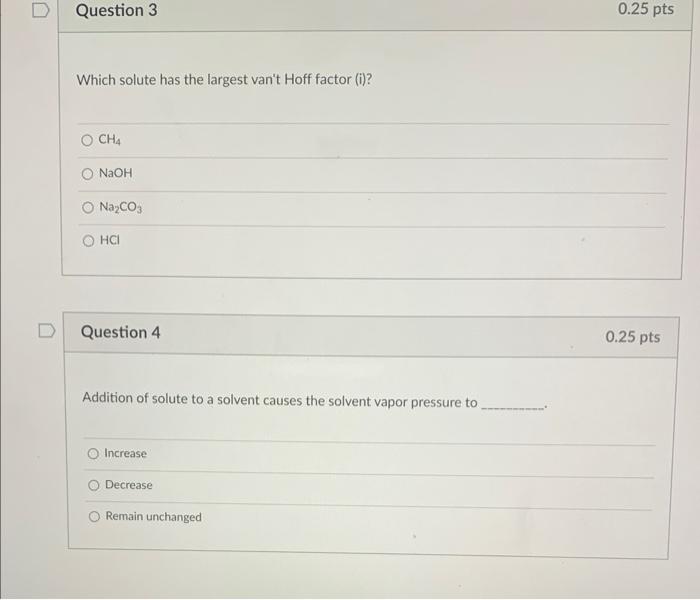

Which solute has the largest van't Hoff factor (i)? CH4NaOHNa2CO3HCl Question 4 Addition of solute to a solvent causes the solvent vapor pressure to Increase Decrease Remain unchanged Calculate H for the reaction 4NH3(s)+5O2(s)4NO(e)+6H2O(8), from the following data. N2(k)+O2(k)2NO(k)N2(k)+3H2(k)2NH3(k)2H2(k)+O2(k)2H2O(k)H=180.5kJH=91.8kJH=483.6kJ 621kJ 756kJ +283kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


