Question: Which solution has the lowest ion concentration (in M)? 0.20 M NH4NO2 0.30 M BaS 0.20 M NaS 0.30 M KMnO4 All of the
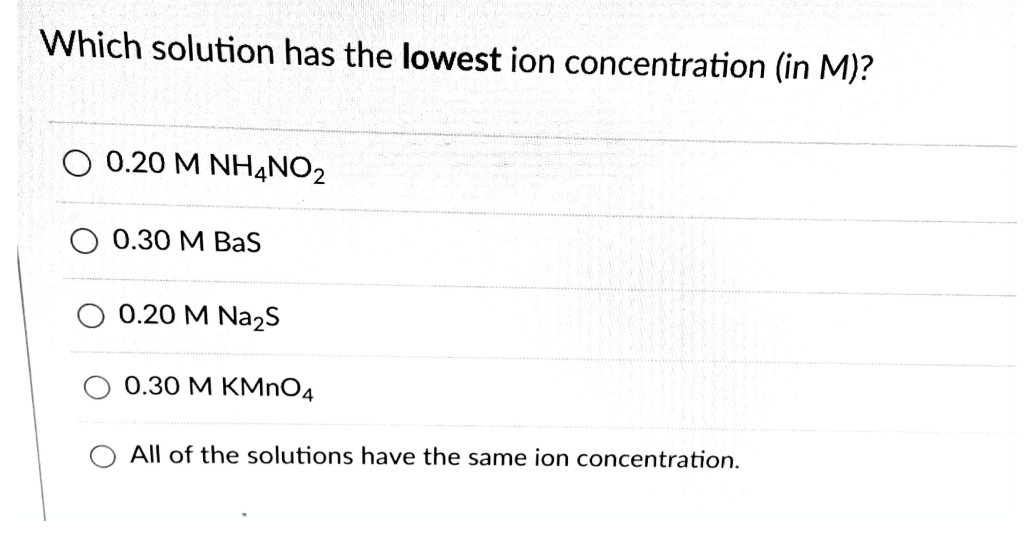
Which solution has the lowest ion concentration (in M)? 0.20 M NH4NO2 0.30 M BaS 0.20 M NaS 0.30 M KMnO4 All of the solutions have the same ion concentration.
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Correct answer 020M NH 4 NO 2 Solution Dissociation happens when a comple... View full answer

Get step-by-step solutions from verified subject matter experts


