Question: Which solution (s) showed the greatest change in pH? Why? which solution showed little or no change in pH? Why? is a buffer supposed to
- Which solution (s) showed the greatest change in pH? Why?
- which solution showed little or no change in pH? Why?
- is a buffer supposed to keep the PH a solution at 7 (neutral)?
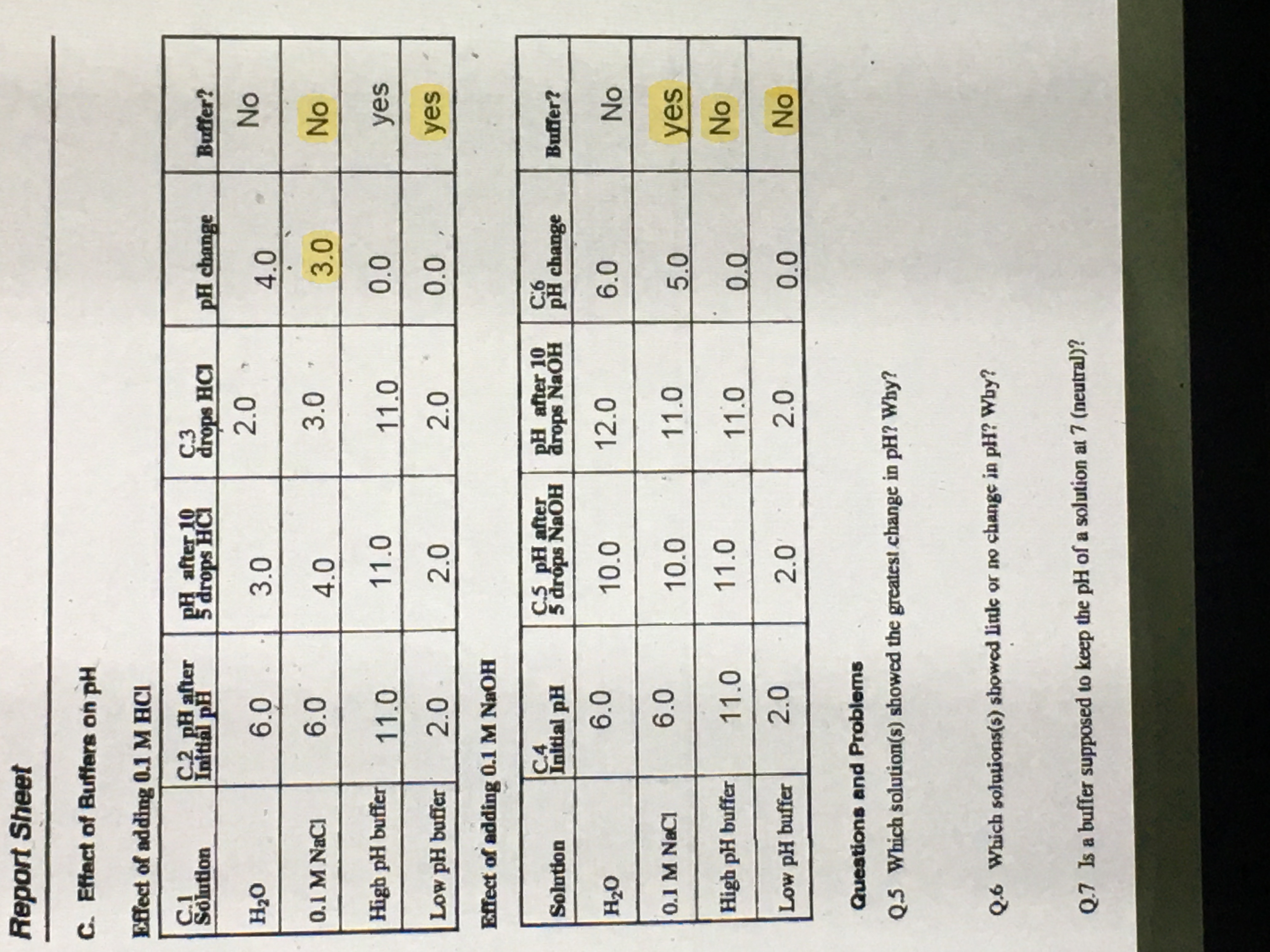 Report Sheet C. Effect of Buffers on pH Effect of adding 0.1 M HCI C.1 Solution C.2 pH after Initial pl PH after 10 5 drops HCI C.3 drops HCI PH change Buffer? H20 6.0 3.0 2.0 4.0 No 0.1 M NaCl 6.0 4.0 3.0 3.0 No High pH buffer 11.0 11.0 11.0 0.0 yes Low PH buffer 2.0 2.0 2.0 0.0 yes Effect of adding 0.1 M NaOH Solution C.4 Initial pH C.S pH after 5 drops NaOH PH after 10 drops NaOH C:6 PH change Buffer? H20 6.0 10.0 12.0 6.0 No 0.1 M NaCl 6.0 10.0 11.0 5.0 yes High pH buffer 11.0 11.0 110 0.0 No Low pH buffer 2.0 2.0 2.0 0.0 No Questions and Problems Q.5 Which solution(s) showed the greatest change in pH? Why? Q.6 Which solutions(s) showed little or no change in pH? Why? Q.7 Is a buffer supposed to keep the pH of a solution at 7 (neutral)
Report Sheet C. Effect of Buffers on pH Effect of adding 0.1 M HCI C.1 Solution C.2 pH after Initial pl PH after 10 5 drops HCI C.3 drops HCI PH change Buffer? H20 6.0 3.0 2.0 4.0 No 0.1 M NaCl 6.0 4.0 3.0 3.0 No High pH buffer 11.0 11.0 11.0 0.0 yes Low PH buffer 2.0 2.0 2.0 0.0 yes Effect of adding 0.1 M NaOH Solution C.4 Initial pH C.S pH after 5 drops NaOH PH after 10 drops NaOH C:6 PH change Buffer? H20 6.0 10.0 12.0 6.0 No 0.1 M NaCl 6.0 10.0 11.0 5.0 yes High pH buffer 11.0 11.0 110 0.0 No Low pH buffer 2.0 2.0 2.0 0.0 No Questions and Problems Q.5 Which solution(s) showed the greatest change in pH? Why? Q.6 Which solutions(s) showed little or no change in pH? Why? Q.7 Is a buffer supposed to keep the pH of a solution at 7 (neutral)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


