Question: Question 4 Consider the following reaction: CH(g) + 2H(g) -> CH6(g) If the instantaneous initial rate of reaction is 1.45 x 10-4 M/s, calculate
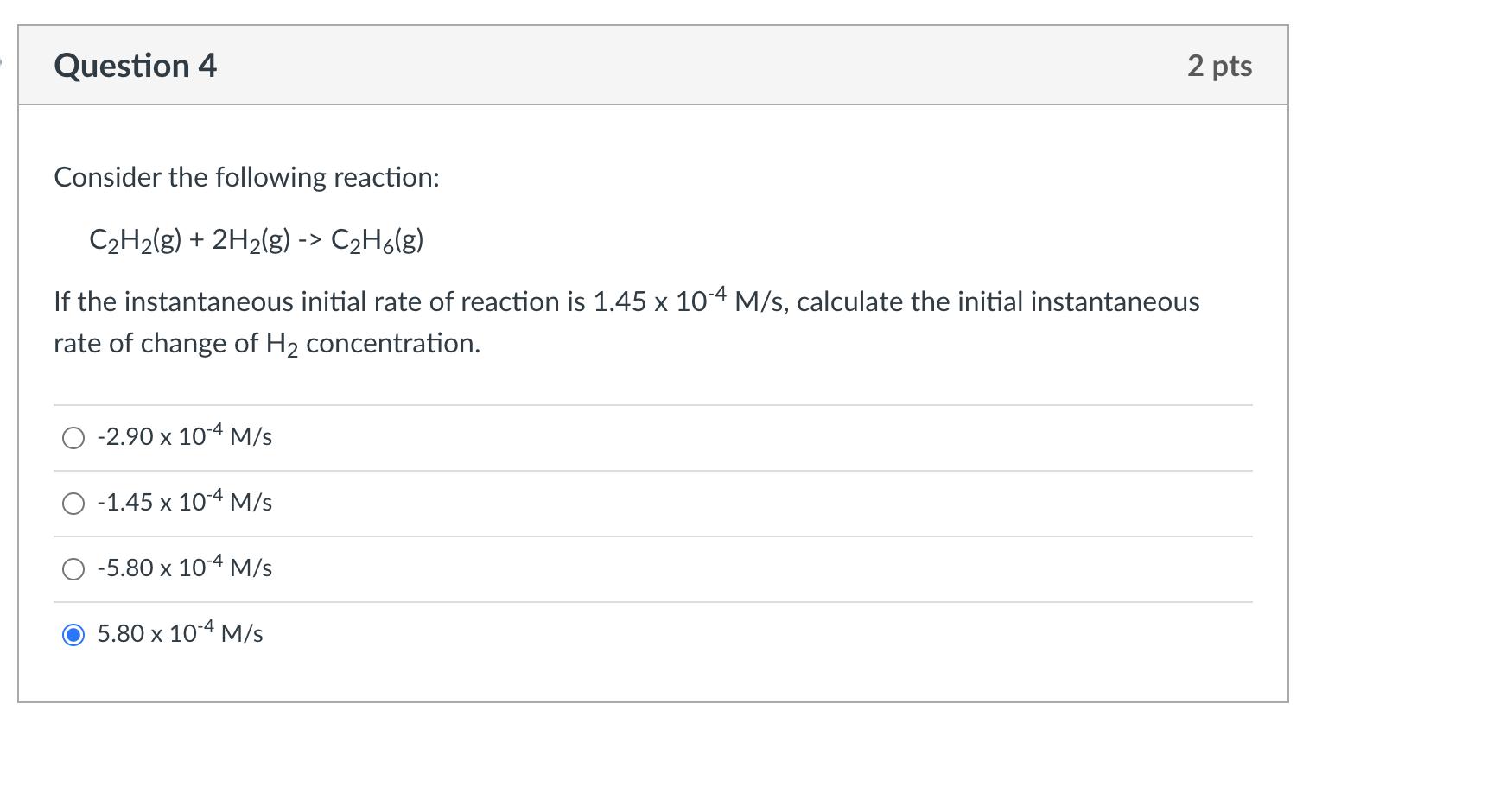
Question 4 Consider the following reaction: CH(g) + 2H(g) -> CH6(g) If the instantaneous initial rate of reaction is 1.45 x 10-4 M/s, calculate the initial instantaneous rate of change of H concentration. -2.90 x 10-4 M/s -1.45 x 10-4 M/s -5.80 x 10-4 M/s 2 pts 5.80 x 10-4 M/s
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Correct option is A As the reaction proceed the ... View full answer

Get step-by-step solutions from verified subject matter experts


