Question: Which statement about Ksp is TRUE? * 1 point The solubility product constant, Ksp, is the value of the equilibrium law equation for a solubility

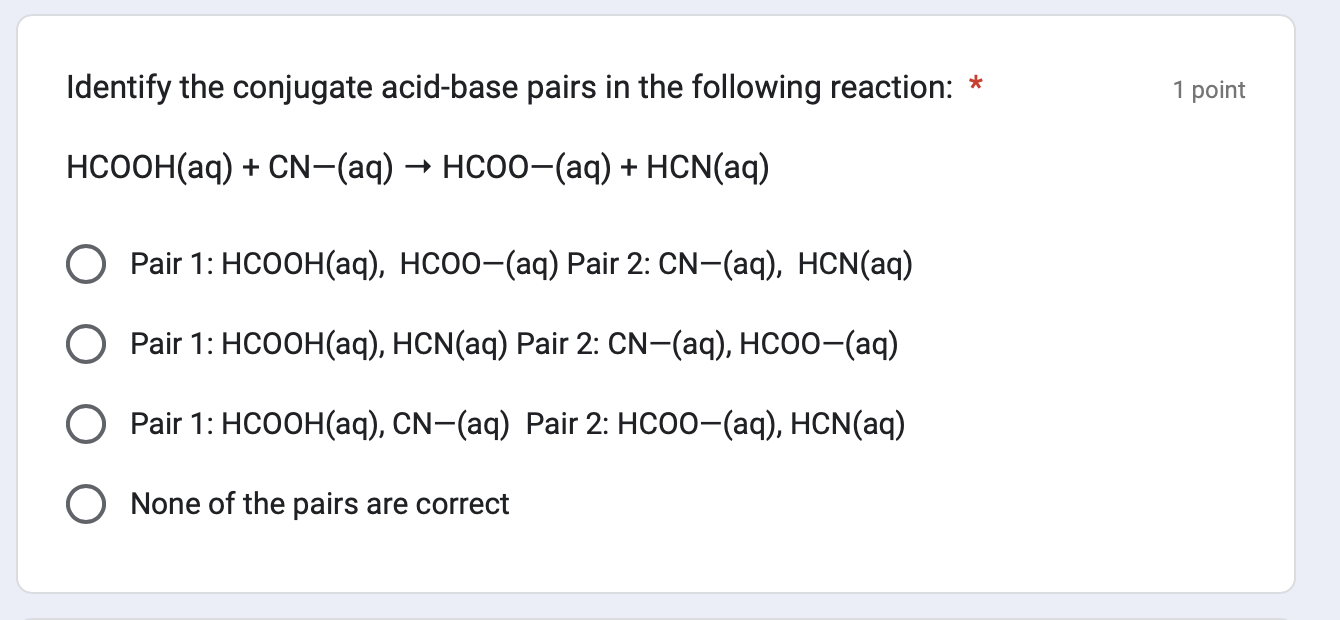
Which statement about Ksp is TRUE? * 1 point The solubility product constant, Ksp, is the value of the equilibrium law equation for a solubility equilibrium and does not include the concentration of the solid. Ksp varies with temperature and the nature of the ionic solid. The solubility product constant, Ksp, is the value of the equilibrium law equation for a solubility equilibrium which also includes the concentration of the solid. Ksp varies with temperature and the nature of the ionic solid. The solubility product constant, Ksp, is the value of the equilibrium law equation for a solubility equilibrium and does not include the concentration of the solid. Ksp resists changing when temperature changes. The solubility product constant, Ksp, is the value of the equilibrium law equation for a solubility equilibrium and does not include the concentration of the solid. Ksp is the same for all ionic solids. Identify the conjugate acid-base pairs in the following reaction: HCOOH(aq)+CN(aq)HCOO(aq)+HCN(aq) Pair 1: HCOOH(aq),HCOO(aq) Pair 2: CN(aq),HCN(aq) Pair 1: HCOOH(aq),HCN(aq) Pair 2: CN(aq),HCOO(aq) Pair 1: HCOOH(aq),CN(aq) Pair 2: HCOO(aq),HCN(aq) None of the pairs are correct
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


