Question: Which statement about Q is FALSE? 1 pts Q changes with temperature. Q is called the reaction quotient. If Q is larger than Ken then
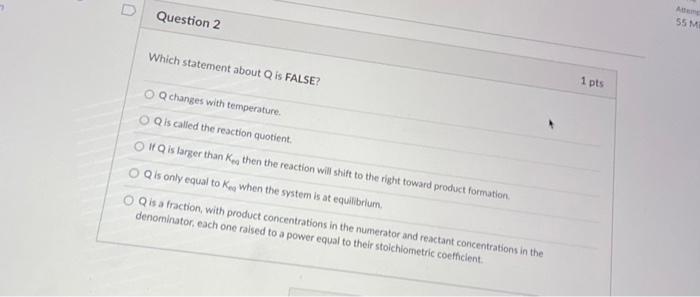
Which statement about Q is FALSE? 1 pts Q changes with temperature. Q is called the reaction quotient. If Q is larger than Ken then the reaction will shift to the right toward product formation. Q is only equai to K. . When Q is a fraction, with product concentrations in the numerator and reactant coricentrations in the denominator, each one raised to a power equal to their stoichiometric coefficient
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


