Question: Which statement is true and which one is false? More hydrophobic solvent has higher eluting power than the hydrophilic solvent when it is used in
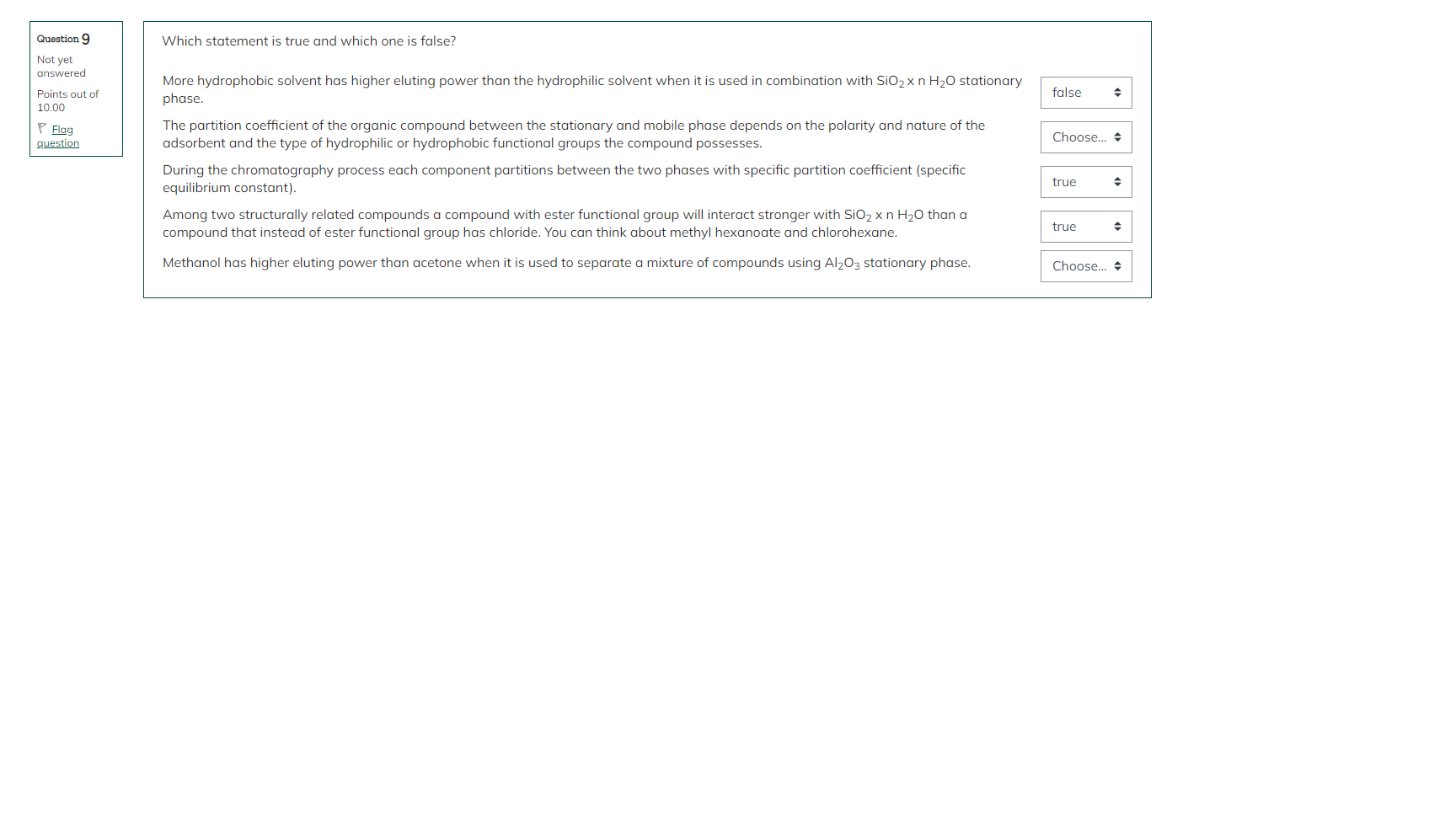
Which statement is true and which one is false? More hydrophobic solvent has higher eluting power than the hydrophilic solvent when it is used in combination with SiO2nH2O stationary phase. The partition coefficient of the organic compound between the stationary and mobile phase depends on the polarity and nature of the adsorbent and the type of hydrophilic or hydrophobic functional groups the compound possesses. During the chromatography process each component partitions between the two phases with specific partition coefficient (specific equilibrium constant). Among two structurally related compounds a compound with ester functional group will interact stronger with SiO2nH2O than a compound that instead of ester functional group has chloride. You can think about methyl hexanoate and chlorohexane. Methanol has higher eluting power than acetone when it is used to separate a mixture of compounds using Al2O3 stationary phase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


