Question: Which Statement Is True Regarding The Heating Curve Below? Note Chat C_3H_8O Has Only One Solid, One Liquid, And One Gas Phase Under The Given
Which Statement Is True Regarding The Heating Curve Below? Note Chat C_3H_8O Has Only One Solid, One Liquid, And One Gas Phase Under The Given Conditions The Inverse Of The Slope Of Line Segment A-B Represents The Molar Heat Capacity Of The Gas. The Temperature Associated With Point D Represents The Melting Point Or - The Substance. The Inverse Of The
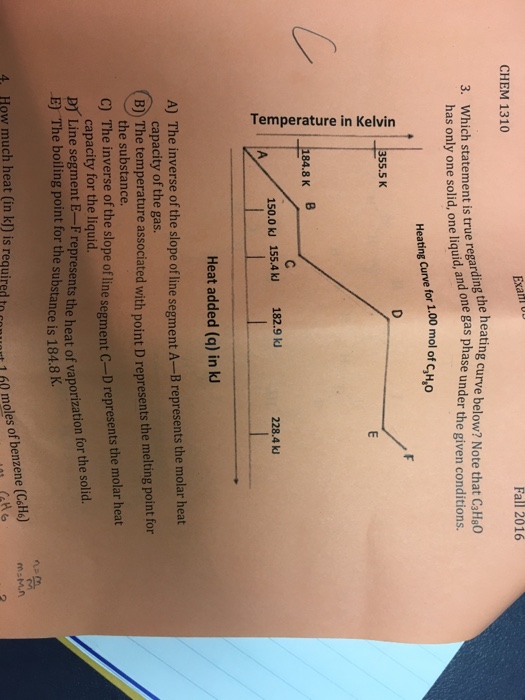
C CHEM 1310 Exa Fall 2016 3. Which statement is true regarding the heating curve below? Note that C3H8O has only one solid, one liquid, and one gas phase under the given conditions. Heating Curve for 1.00 mol of CHO Temperature in Kelvin 355.5 K 184.8 K B D 150.0 kJ 155.4 kJ 182.9 kJ 228.4 kJ A Heat added (q) in kJ A) The inverse of the slope of line segment A-B represents the molar heat capacity of the gas. B) The temperature associated with point D represents the melting point for the substance. C) The inverse of the slope of line segment C-D represents the molar heat capacity for the liquid. E) The boiling point for the substance is 184.8 K. D Line segment E-F represents the heat of vaporization for the solid. 4. How much heat (in kJ) is required to convert 1.60 moles of benzene (C6H6) Catto M=Mn 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


