Question: While each molecule below has more protons ( H-atoms) than being shown, just focus on the protons identified. Compare the two protons identified. 1) Draw

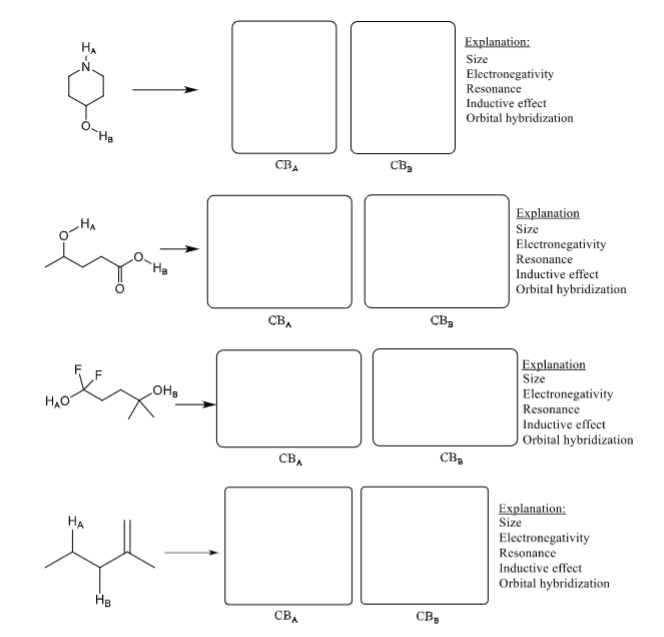
While each molecule below has more protons ( H-atoms) than being shown, just focus on the protons identified. Compare the two protons identified. 1) Draw the two conjugate bases that could possibly result (although one will be preferred over the other). The purpose here is to reinforce the idea that you should look at the conjugate base and the atom upon which the negative charge resides, then you should consider your SERIO factors. 2) Identify which proton is more acidic by circling the Ha or Hb on the original molecule. 3) Explain why by comparing the conjugate bases. Circle the factor you considered when comparing the stabilities of the conjugate bases. Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


