Question: While that doesn't seem like a large change, small changes in pH can represent larger and larger changes in the H* ion concentration, which can
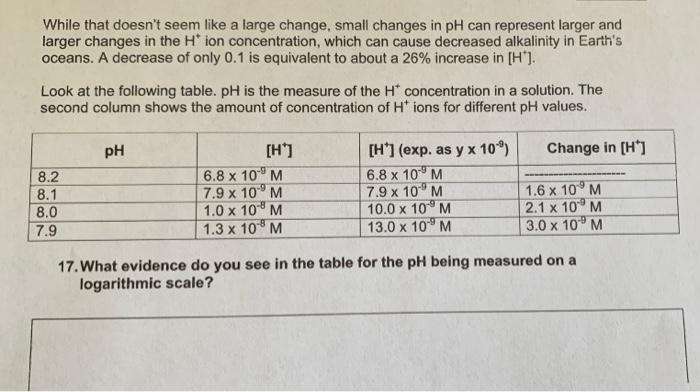
While that doesn't seem like a large change, small changes in pH can represent larger and larger changes in the H* ion concentration, which can cause decreased alkalinity in Earth's oceans. A decrease of only 0.1 is equivalent to about a 26% increase in [H"). Look at the following table. pH is the measure of the H* concentration in a solution. The second column shows the amount of concentration of Ht ions for different pH values. pH Change in [H] 8.2 8.1 8.0 7.9 [H] 6.8 x 10-'M 7.9 x 10M 1.0 x 10M 1.3 x 10 PM [HT] (exp. as y x 10) 6.8 x 10 7.9 x 10-'M 10.0 x 10M 13.0 x 10M 1.6 x 10M 2.1 x 10PM 3.0 x 100 M 17. What evidence do you see in the table for the pH being measured on a logarithmic scale
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


