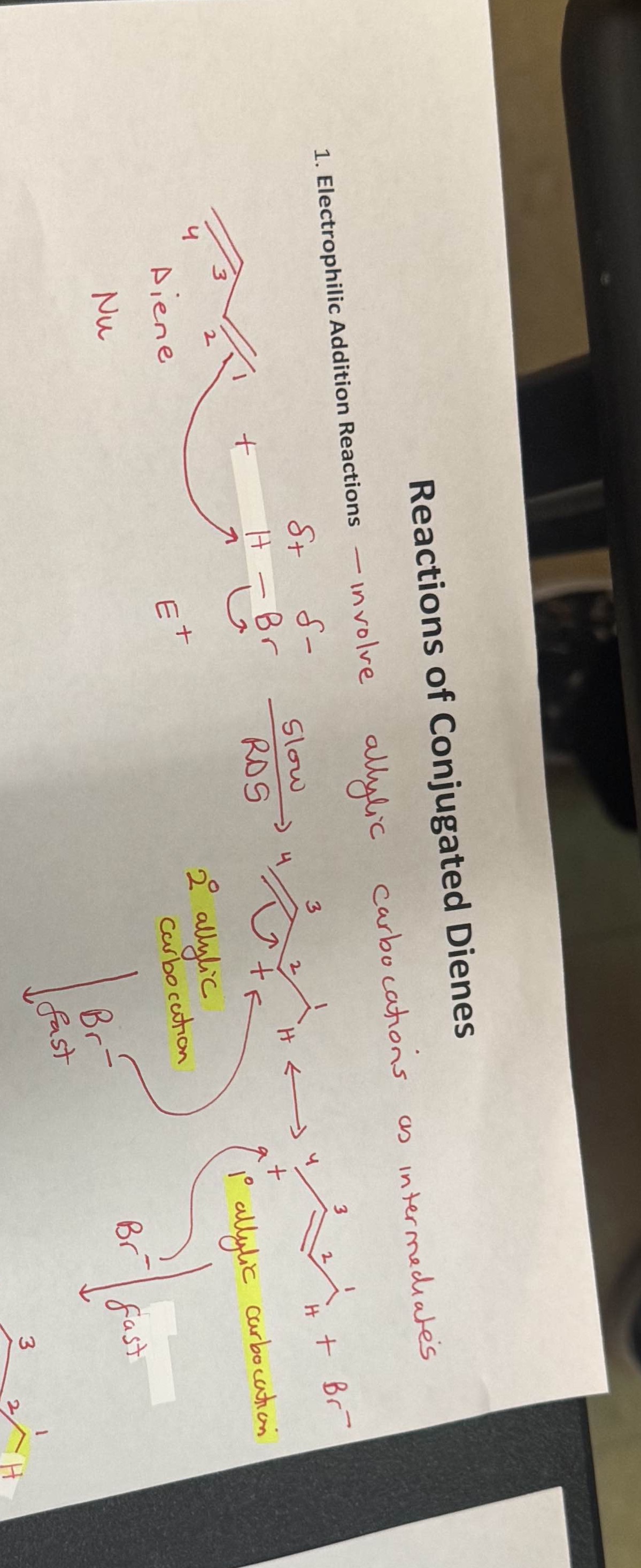
Question: Why did we move the double bond from in between carbon 3-4 to 2-3 is it because it is more stable near the carbocation? Reactions
Why did we move the double bond from in between carbon 3-4 to 2-3 is it because it is more stable near the carbocation?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


