Question: Why? Equilibrium constants such as Kc and Ksp describe solid dissolution and precipitation equilibria. Experimental data can be collected to calculate the values of these
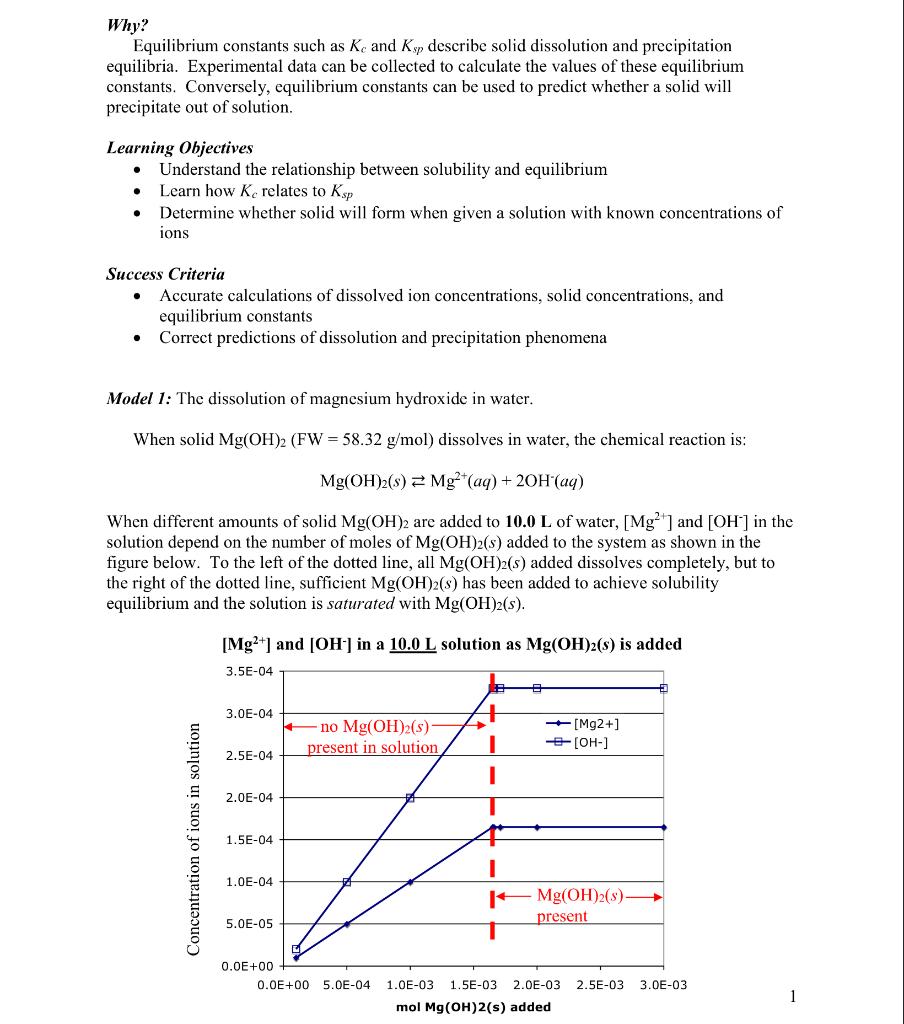
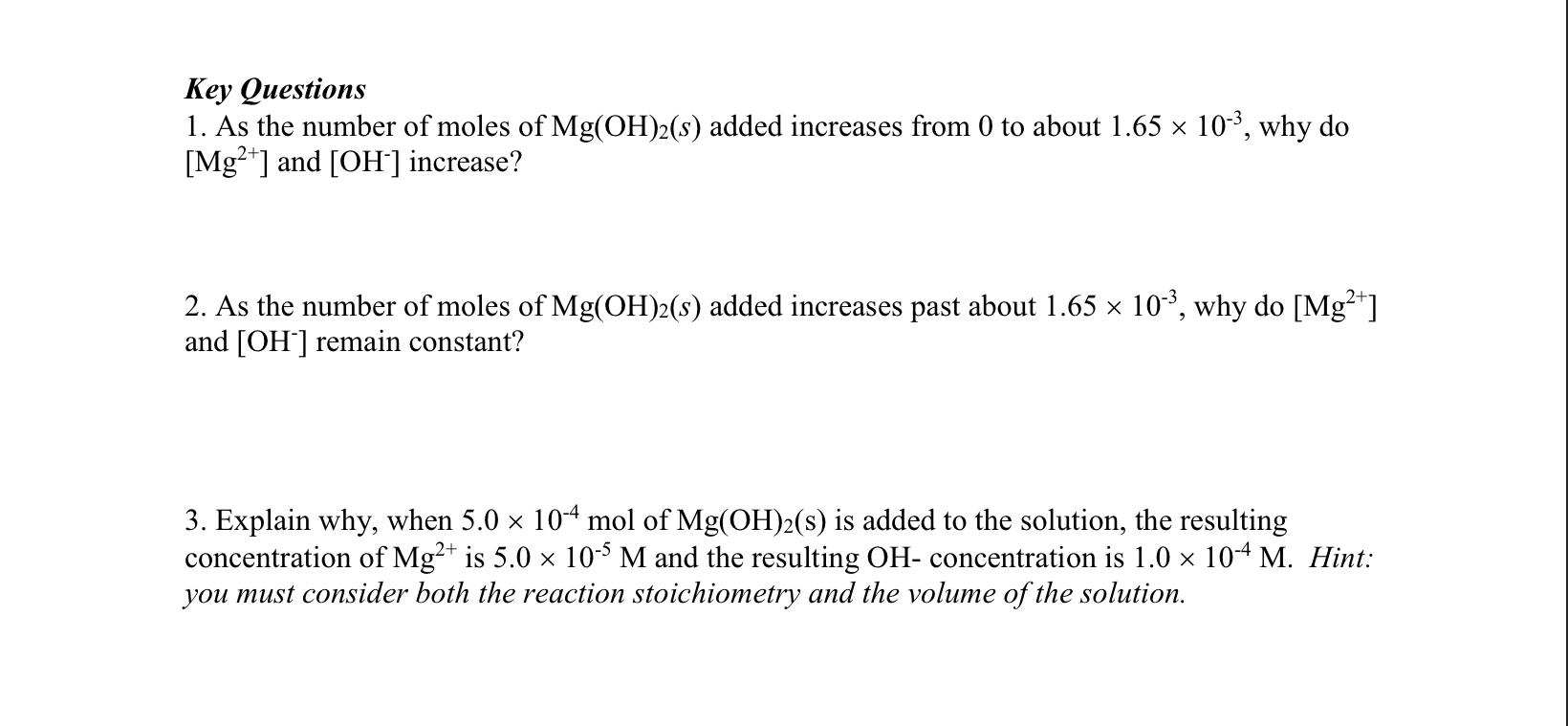
Why? Equilibrium constants such as Kc and Ksp describe solid dissolution and precipitation equilibria. Experimental data can be collected to calculate the values of these equilibrium constants. Conversely, equilibrium constants can be used to predict whether a solid will precipitate out of solution. Learning Objectives - Understand the relationship between solubility and equilibrium - Learn how Kc relates to Ksp - Determine whether solid will form when given a solution with known concentrations of ions Success Criteria - Accurate calculations of dissolved ion concentrations, solid concentrations, and equilibrium constants - Correct predictions of dissolution and precipitation phenomena Model 1: The dissolution of magnesium hydroxide in water. When solid Mg(OH)2(FW=58.32g/mol) dissolves in water, the chemical reaction is: Mg(OH)2(s)Mg2+(aq)+2OH(aq) When different amounts of solid Mg(OH)2 are added to 10.0L of water, [Mg2+] and [OH]in the solution depend on the number of moles of Mg(OH)2(s) added to the system as shown in the figure below. To the left of the dotted line, all Mg(OH)2(s) added dissolves completely, but to the right of the dotted line, sufficient Mg(OH)2(s) has been added to achieve solubility equilibrium and the solution is saturated with Mg(OH)2(s). [Mg2+] and [OH]in a 10.0L solution as Mg(OH)2(s) is added Key Questions 1. As the number of moles of Mg(OH)2(s) added increases from 0 to about 1.65103, why do [Mg2+] and [OH]increase? 2. As the number of moles of Mg(OH)2(s) added increases past about 1.65103, why do [Mg2+] and [OH]remain constant? 3. Explain why, when 5.0104mol of Mg(OH)2(s) is added to the solution, the resulting concentration of Mg2+ is 5.0105M and the resulting OH - concentration is 1.0104M. Hint: you must consider both the reaction stoichiometry and the volume of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


