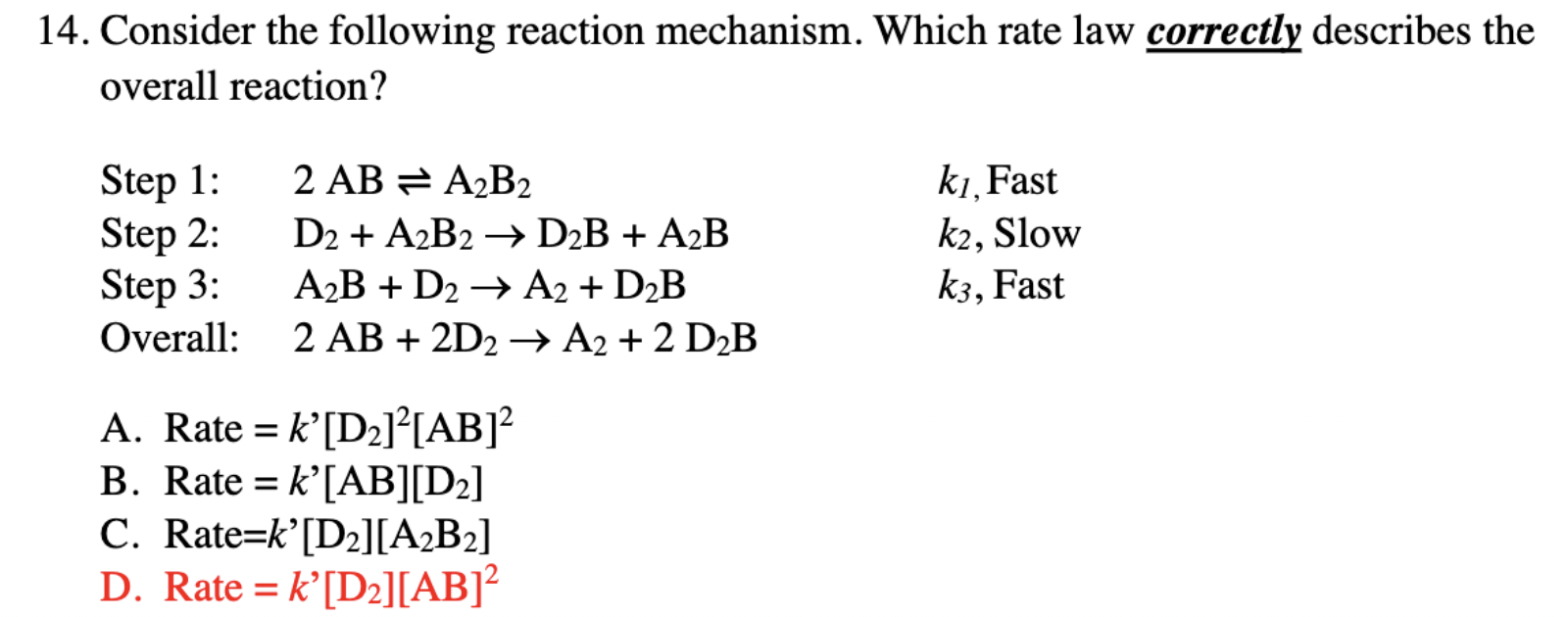
Question: Why is it D and not C? 14. Consider the following reaction mechanism. Which rate law correctly describes the overall reaction? Step 1: 2ABA2B2k1, Fast
 Why is it D and not C?
Why is it D and not C?
14. Consider the following reaction mechanism. Which rate law correctly describes the overall reaction? Step 1: 2ABA2B2k1, Fast Step 2: D2+A2B2D2B+A2Bk2, Slow Step 3: A2B+D2A2+D2Bk3, Fast Overall: 2AB+2D2A2+2D2B A. Rate =k[D2]2[AB]2 B. Rate =k[AB][D2] C. Rate =k ' [D2][A2B2] D. Rate =k[D2][AB]2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


