Question: will rate Solve these two problems using a Freundlich isotherm with the following parameters: K= 1.2x102 and 1 = 0.1 (concentrations in mg/L) a. Direct
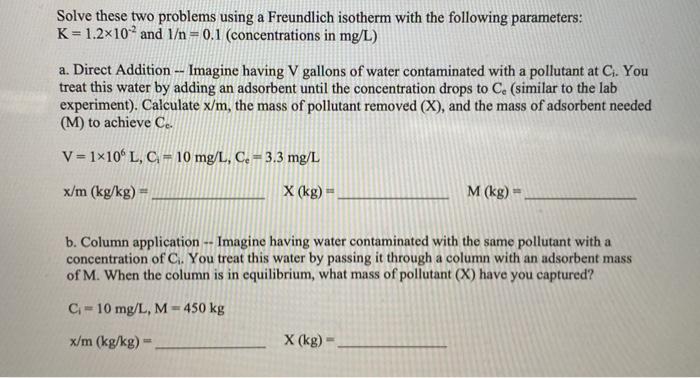
Solve these two problems using a Freundlich isotherm with the following parameters: K= 1.2x102 and 1 = 0.1 (concentrations in mg/L) a. Direct Addition -- Imagine having V gallons of water contaminated with a pollutant at C. You treat this water by adding an adsorbent until the concentration drops to C. (similar to the lab experiment). Calculate x/m, the mass of pollutant removed (X), and the mass of adsorbent needed (M) to achieve C. m V = 1x10 L, C = 10 mg/L, C. = 3.3 mg/L x/m (kg/kg) - X (kg) - M (kg) b. Column application -- Imagine having water contaminated with the same pollutant with a concentration of C. You treat this water by passing it through a column with an adsorbent mass of M. When the column is in equilibrium, what mass of pollutant (X) have you captured? C-10 mg/L, M-450 kg x/m (kg/kg) X (kg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


