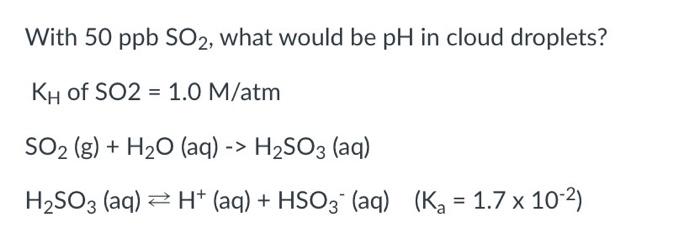
Question: With 50 ppb SO2, what would be pH in cloud droplets? KH of SO2 = 1.0 M/atm SO2 (g) + H2O (aq) -> H2SO3 (aq)
With 50 ppb SO2, what would be pH in cloud droplets?
KH of SO2 = 1.0 M/atm
SO2 (g) + H2O (aq) -> H2SO3 (aq)
H2SO3 (aq) H+ (aq) + HSO3- (aq) (Ka = 1.7 x 10-2)
KH of SO2=1.0M/atm SO2(g)+H2O(aq)>H2SO3(aq)H2SO3(aq)H+(aq)+HSO3(aq)(Ka=1.7102)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


