Question: With the following data, calculate the pre-exponential factor (A) and the activation energy (Ea): T(K): 500.0 520.0 560.0 600.0 CA(gmol/cm3): 0.1442E-02 0.2357E-03 0.1316E-03 0.7574E-04 With
With the following data, calculate the pre-exponential factor (A) and the activation energy (Ea):
T(K): 500.0 520.0 560.0 600.0
CA(gmol/cm3): 0.1442E-02 0.2357E-03 0.1316E-03
0.7574E-04
With the equation of the reactor (CTR), we clear the value of K*
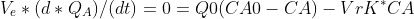 getting like this
getting like this
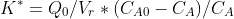
Get the values of Ea and A, please.
Qo=100 cm3/min
Vr=1500 cm3
Ca0=0.005 gmol/cm3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


