Question: Without doing a calculation, predict whether the entropy change will be positive or negative when each of the following reactions occurs in the direction it
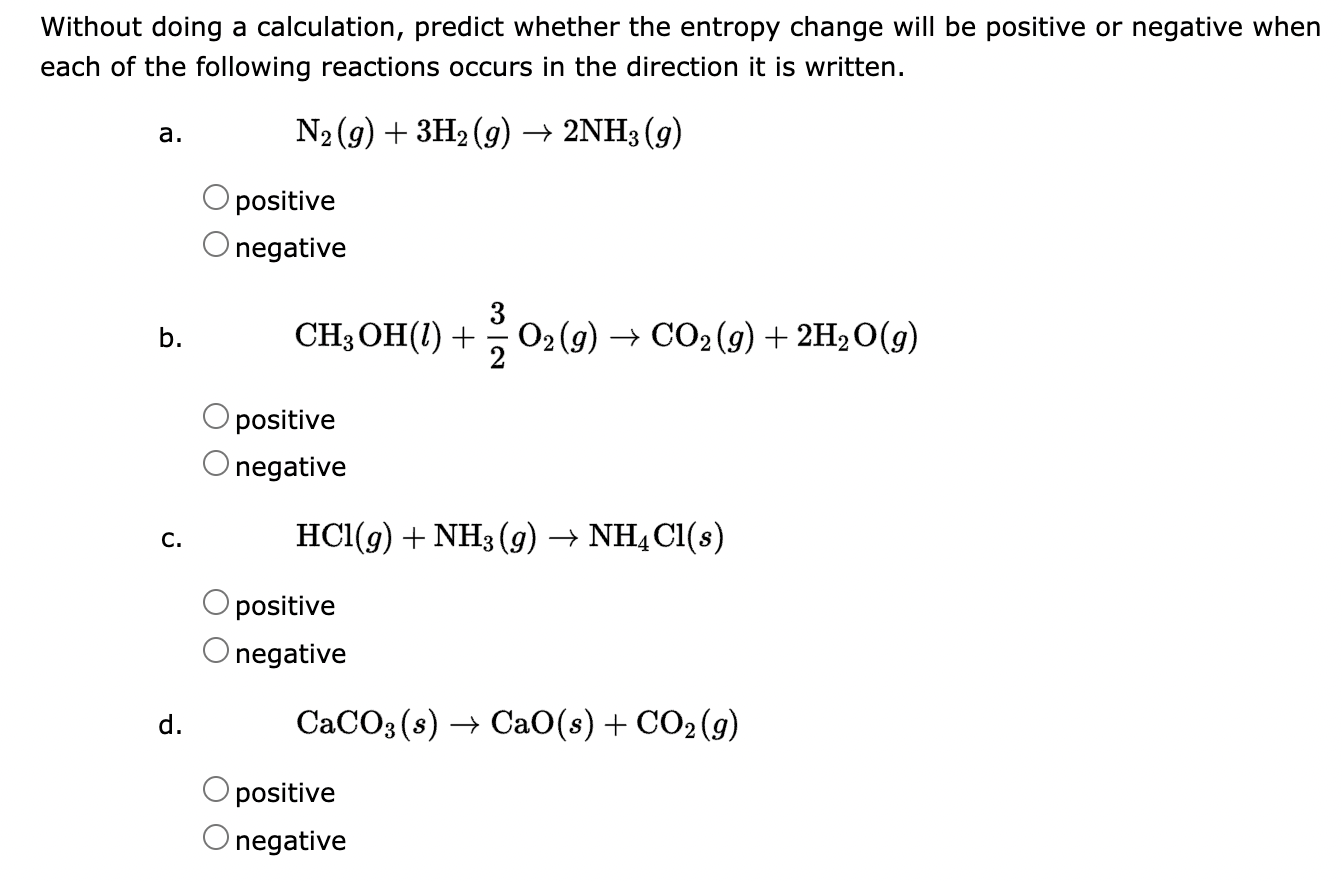
Without doing a calculation, predict whether the entropy change will be positive or negative when each of the following reactions occurs in the direction it is written. a. N2(g)+3H2(g)2NH3(g) positive negative b. CH3OH(l)+23O2(g)CO2(g)+2H2O(g) positive negative c. HCl(g)+NH3(g)NH4Cl(s) positive negative d. CaCO3(s)CaO(s)+CO2(g) positive negative
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


