Question: Without using a Ka table, predict the position of equilibrium for the given reaction, and explain your choice. The forward reaction is favored because oxygen
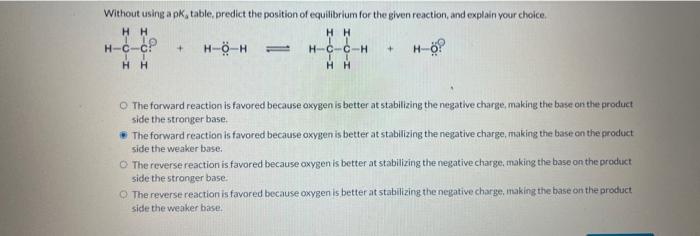
Without using a Ka table, predict the position of equilibrium for the given reaction, and explain your choice. The forward reaction is favored because oxygen is better at stabilizing the negative charge, making the base on the product side the stronger base. The forward reaction is favored because oxygen is better at stabilizing the negative charge, making the base on the product side the weaker base. The reverse reaction is favored because oxygen is better at stabilizing the negative charge, making the base on the product side the stronger base: The reverse reaction is fayored bocause oxygen is better at stabilizing the negative charge, inaking the base on the product side the weaker hase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


