Question: WM RIO- Experiment 11 - Molecular Structure amples Nec 1. 2 3.0-09-2.1 Ionic bonding Crystal lattice present 3 through 8 will not be done for

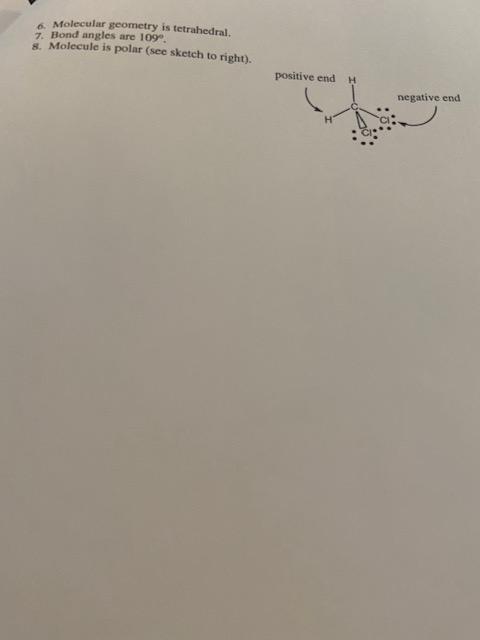


WM RIO- Experiment 11 - Molecular Structure amples Nec 1. 2 3.0-09-2.1 Ionic bonding Crystal lattice present 3 through 8 will not be done for this is an one compound 3.5 -2.1 1.4 polar covalent bonding 2. True molecule (dot structure will be drawn in 19) E HO 1. 3. -H 4. Model has 2 yellow balls and one red ball and 4 sticks (two sticks are for the single bonds between the oxygen and the hydrogens and the other sticks are used for the non-bonding electron pairs around the oxygen.) 5. Electrons around the central atom (oxygen) are tetrahedrally arranged 6. Molecule has a bent or angular geometry. 7. Bond angles are approximately 109 8. Molecule is polar (see sketch to the right). negative end positive end CH.CH 1. Electronegativity difference: 3.0 higher electronegativity -2.1 lower electronegativity 0.9 electronegativity difference 2. True molecule 3. :ci: H-C-H :: 4. Model has central black ball (C), 2 yellow balls (H), 2 green balls (CI). 4 sticks are used for single bonds to the central C. 6 sticks are used for non-bonding pairs of electrons around the two chlorines (CI). 5. Electronic geometry is tetrahedral. Molecular geometry is tetrahedral. 7. Bond angles are 109 8. Molecule is polar (see sketch to right). positive end negative end CHEM R110 Name Experiment 11-Molecular Models-Report Sheet For each of the molecules, ions or ionic compounds below, follow the 8 steps given to you on pages 62 and 63 of this experiment. 1. Br2 2. HCI 3. NaF 4. CCL 5. NO2 6. PH 7. CH2 (Describe the geometry about each central carbon atom.) 8. CO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


