Question: Work this problem out on paper first, then answer the following questions. Given this balanced reaction: Mn2O3(s) + 3 CO(g) 2 Mn(s) + 3 CO2(g)
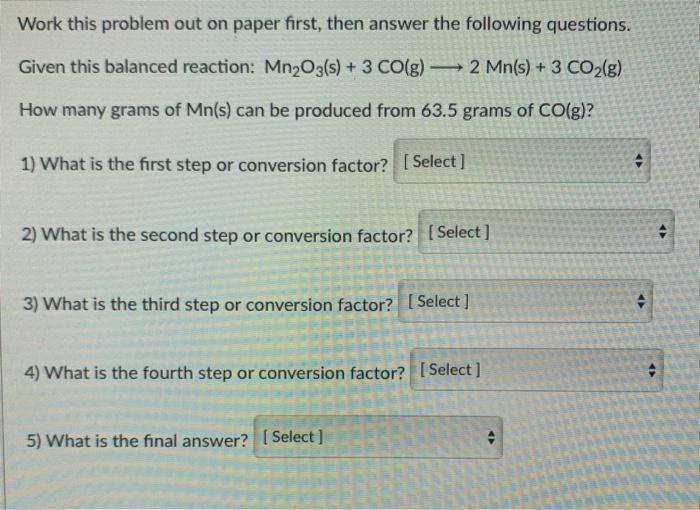
Work this problem out on paper first, then answer the following questions. Given this balanced reaction: Mn2O3(s) + 3 CO(g) 2 Mn(s) + 3 CO2(g) How many grams of Mn(s) can be produced from 63.5 grams of CO(g)? 1) What is the first step or conversion factor? [Select] 2) What is the second step or conversion factor? [Select] 4 3) What is the third step or conversion factor? [Select] 4) What is the fourth step or conversion factor? [Select] 5) What is the final answer? (Select]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


