Question: would need help with this question: PLEASE put in excel form on plotting the graph And write equation when its ONLY A STRAIGHT LINE. Also
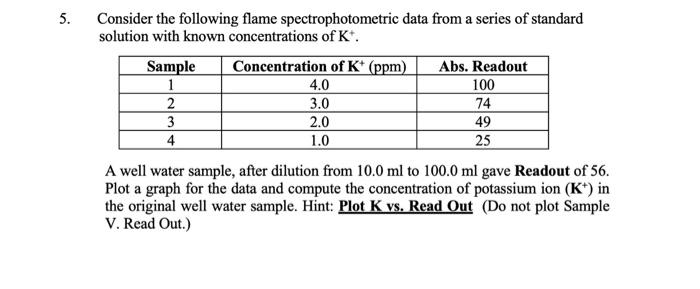
5. Consider the following flame spectrophotometric data from a series of standard solution with known concentrations of K. Sample Concentration of K+ (ppm) Abs. Readout 1 4.0 100 2 3.0 74 3 2.0 49 4 1.0 25 A well water sample, after dilution from 10.0 ml to 100.0 ml gave Readout of 56. Plot a graph for the data and compute the concentration of potassium ion (K*) in the original well water sample. Hint: Plot K vs. Read Out (Do not plot Sample V. Read Out.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


