Question: Would this answer be correct? A student wants to standardize their thiosulfate solution with KlO3. They needed 13.40 mL of S2O32 solution to neutralire 18.0
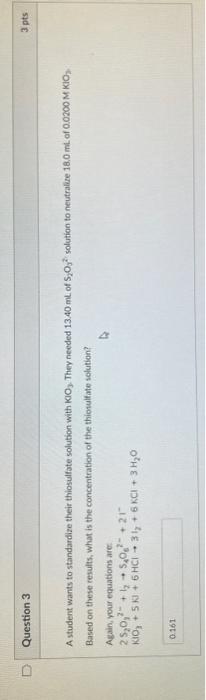
A student wants to standardize their thiosulfate solution with KlO3. They needed 13.40 mL of S2O32 solution to neutralire 18.0 mL of 0.0200 M KiO, Based on these results, What is the concentration of the thiouslfate solutian? Again, your equations are: 2S2O32+l254O62+2lKiO3+5KJ+6HCl3ly+6KCl+3H2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


