Question: Would you explain why it comes out to 92/662 instead of 184/662? I thought it would be 184 instead of 92 because it's 4NO 2
Would you explain why it comes out to 92/662 instead of 184/662? I thought it would be 184 instead of 92 because it's 4NO2 not 2NO2
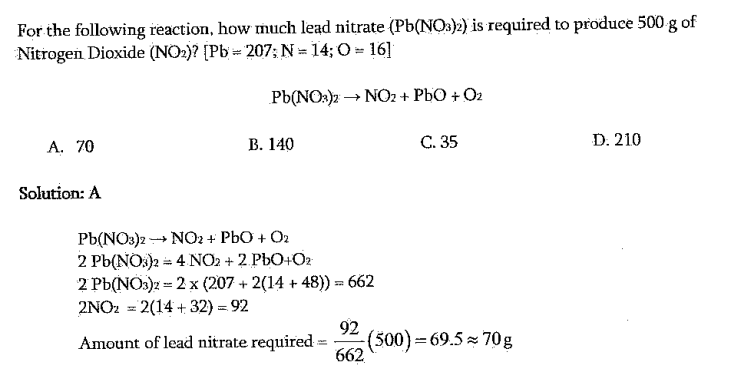
For the following reaction, how much lead nitrate (Pb(NO3)2) is required to produce 500g of Nitrogen Dioxide (NO2)?[Pb=207;N=14;O=16] Pb(NO3)2NO2+PbO+O2 A. 70 B. 140 C. 35 D. 210 Solution: A Pb(NO3)2NO2+PbO+O22Pb(NO)2=4NO2+2PbO2O22Pb(NO3)2=2(207+2(14+48))=6622NO2=2(14+32)=92Amountofleadnitraterequired=66292(500)=69.570g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


