Question: Write a MATLAB code for the following question. I DO NOT need a handwritten solution. If you can't do Matlab let someone else do it
Write a MATLAB code for the following question. I DO NOT need a handwritten solution. If you can't do Matlab let someone else do it do not waste my question i needed in matlab as a code only
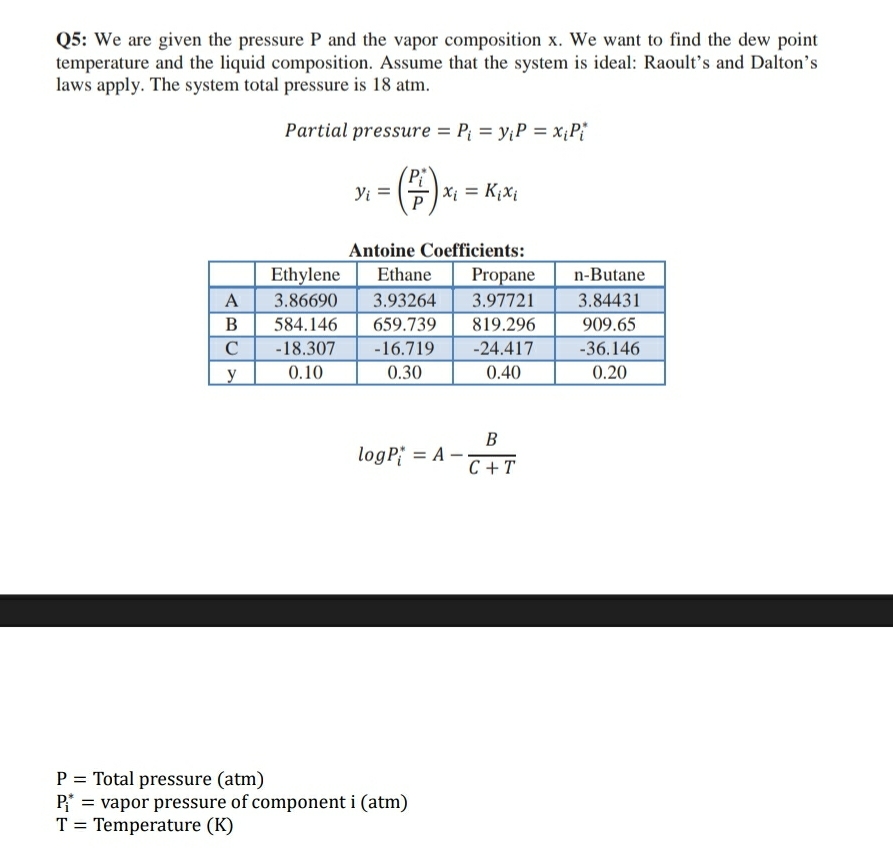
Q: We are given the pressure and the vapor composition We want to find the dew point temperature and the liquid composition. Assume that the system is ideal: Raoult's and Dalton's laws apply. The system total pressure is atm. vapor composition is
Partial pressure
Antoine Coefficients:
tableEthylene,Ethane,Propane,nButaneABCy
Total pressure atm
vapor pressure of component
Temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


