Question: Write a net ionic equation to show why the solubility of Pb(OH)2(s) increases in the presence of a strong acid and calculate the equilibrium
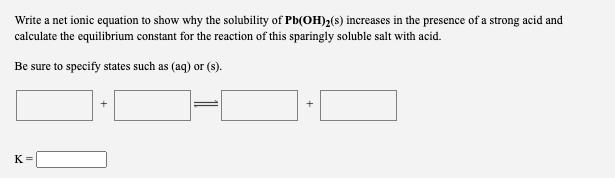
Write a net ionic equation to show why the solubility of Pb(OH)2(s) increases in the presence of a strong acid and calculate the equilibrium constant for the reaction of this sparingly soluble salt with acid. Be sure to specify states such as (aq) or (s). + + K=
Step by Step Solution
There are 3 Steps involved in it
To find a balanced net ionic equation to show why the solubility of PbOH2s increases in the presen... View full answer

Get step-by-step solutions from verified subject matter experts


