Question: Suppose a student performed a similar experiment with a different unknown. Given the data in the table, what is the van't Hoff factor of
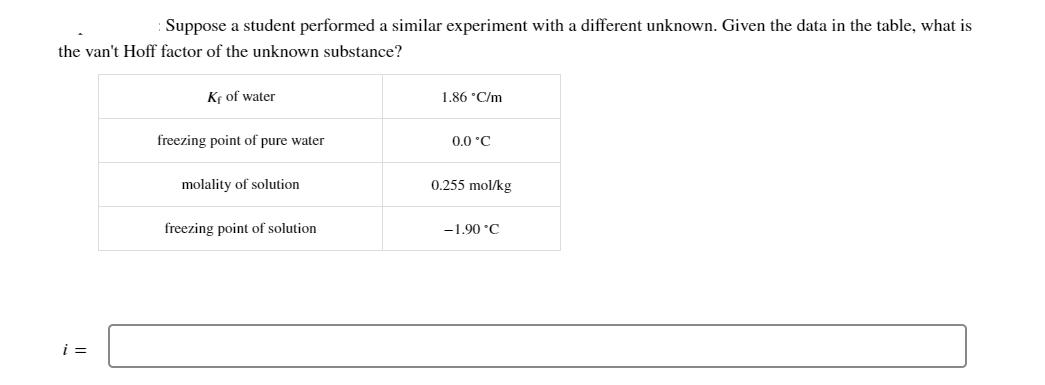
Suppose a student performed a similar experiment with a different unknown. Given the data in the table, what is the van't Hoff factor of the unknown substance? i = Kr of water freezing point of pure water molality of solution freezing point of solution 1.86 C/m 0.0 C 0.255 mol/kg -1.90 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


