Question: Write down the given systems MgO-Al 2 O 3 and Mg-Pb . The following questions refer to these phase diagrams shown here: 2. (3
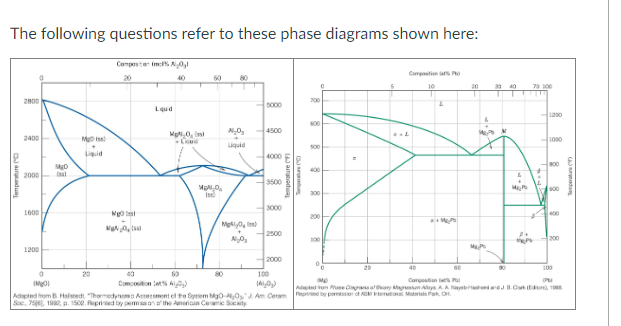
- Write down the given systems "MgO-Al2O3 and Mg-Pb".
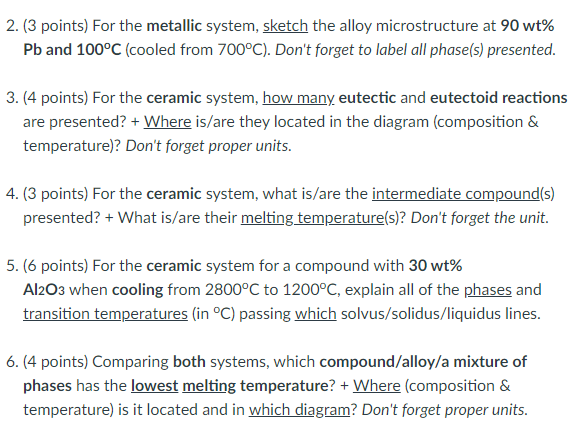
The following questions refer to these phase diagrams shown here: 2. (3 points) For the metallic system, sketch the alloy microstructure at 90wt% Pb and 100C (cooled from 700C ). Don't forget to label all phase(s) presented. 3. (4 points) For the ceramic system, how many eutectic and eutectoid reactions are presented? + Where is/are they located in the diagram (composition \& temperature)? Don't forget proper units. 4. (3 points) For the ceramic system, what is/are the intermediate compound(s) presented? + What is/are their melting temperature(s)? Don't forget the unit. 5. (6 points) For the ceramic system for a compound with 30wt% Al2O3 when cooling from 2800C to 1200C, explain all of the phases and transition temperatures (in C ) passing which solvus/solidus/liquidus lines. 6. (4 points) Comparing both systems, which compound/alloy/a mixture of phases has the lowest melting temperature? +Where(composition& temperature) is it located and in which diagram? Don't forget proper units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


