Question: Write down the steps or the algorithm involved in the calculation BUBL T for a binary system using Modified Raoult's law. For the system of
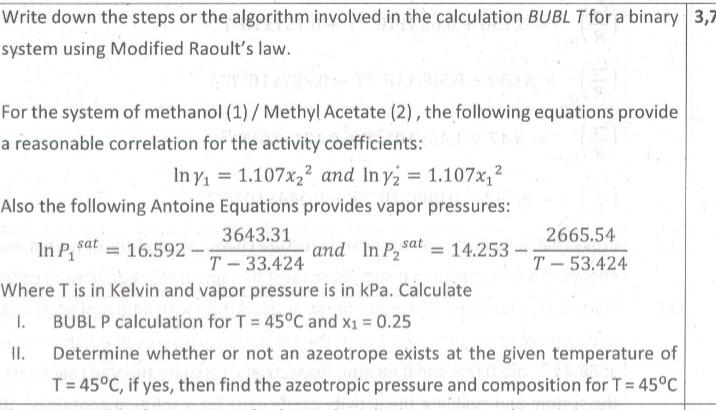
Write down the steps or the algorithm involved in the calculation BUBL T for a binary system using Modified Raoult's law. For the system of methanol (1) / Methyl Acetate (2) , the following equations provide a reasonable correlation for the activity coefficients: ln1=1.107x22andln2=1.107x12 Also the following Antoine Equations provides vapor pressures: lnP1sat=16.592T33.4243643.31andlnP2sat=14.253T53.4242665.54 Where T is in Kelvin and vapor pressure is in kPa. Calculate I. BUBL P calculation for T=45C and x1=0.25 II. Determine whether or not an azeotrope exists at the given temperature of T=45C, if yes, then find the azeotropic pressure and composition for T=45C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


