Question: Write the anodic, cathodic and net cell reactions, calculate the EMF, AG of the cell and state whether the cell can act as galvanic cell
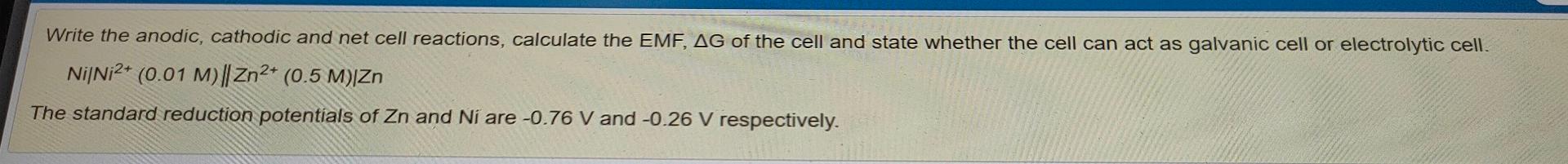
Write the anodic, cathodic and net cell reactions, calculate the EMF, AG of the cell and state whether the cell can act as galvanic cell or electrolytic cell. Ni/Ni2+ (0.01 M)|| Zn2+ (0.5 M) Zn The standard reduction potentials of Zn and Ni are -0.76 V and -0.26 V respectively
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


