Question: Write the empirical formula for at least four ionic compounds that could be formed from the following ions: NH, Cro , co Ph** 4+ 0
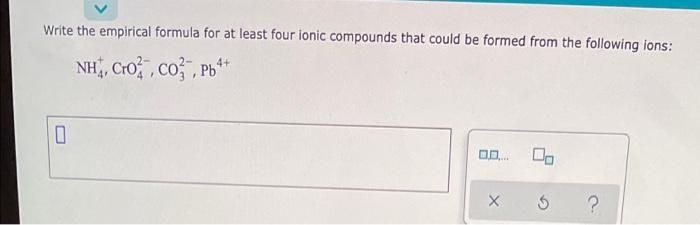
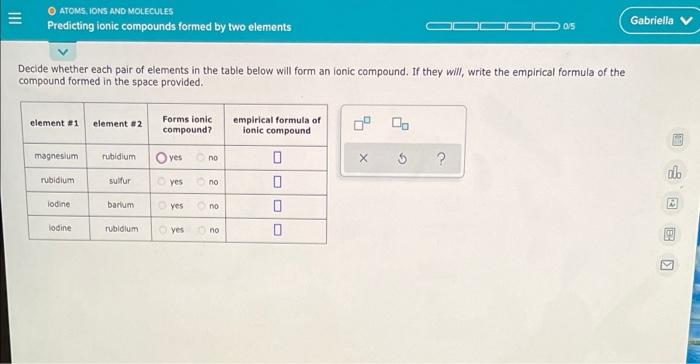
Write the empirical formula for at least four ionic compounds that could be formed from the following ions: NH, Cro , co Ph** 4+ 0 X 5 ? ATOMS, IONS AND MOLECULES Predicting ionic compounds formed by two elements 05 Gabriella v Decide whether each pair of elements in the table below will form an lonic compound. If they will write the empirical formula of the compound formed in the space provided. element #1 element #2 Forms ionic compound? empirical formula of ionic compound magnesium rubidium Oves no . $ ? allo rubidium sulfur yes no 0 lodine barium yes no 0 lodine rubidium yes no
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


