Question: Write the ionization equilibria for this tripeptide. What would be the net charge of this tripeptide at pH 7? backbone R2 1.45A pepide plane
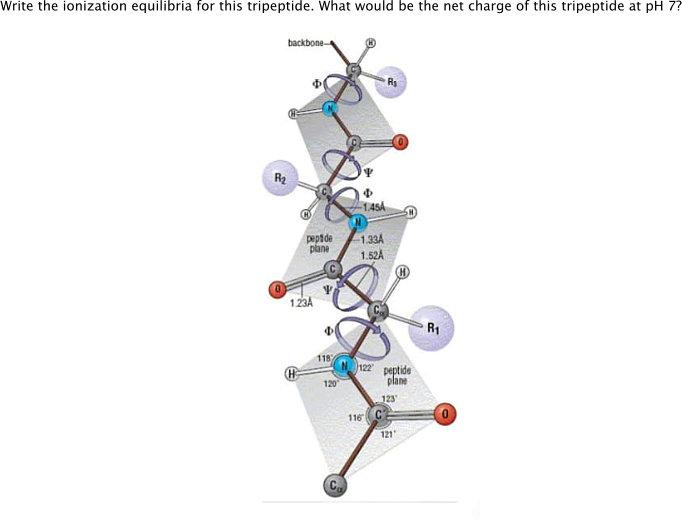
Write the ionization equilibria for this tripeptide. What would be the net charge of this tripeptide at pH 7? backbone R2 1.45A pepide plane 1.33A 1.52A 123A R1 118 122 peptide plane 120 123 116 121
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
1A tripeptide composed of three different amino acids can be made in 6 diff... View full answer

Get step-by-step solutions from verified subject matter experts


