Question: Write the line notation for the following cell. Pt HBr(aq, 0.10 M) Bra() Al(NO3)3(aq, 0.010 M) Line notation: Line notation: Answer Bank AI(NO), (aq, 0.010
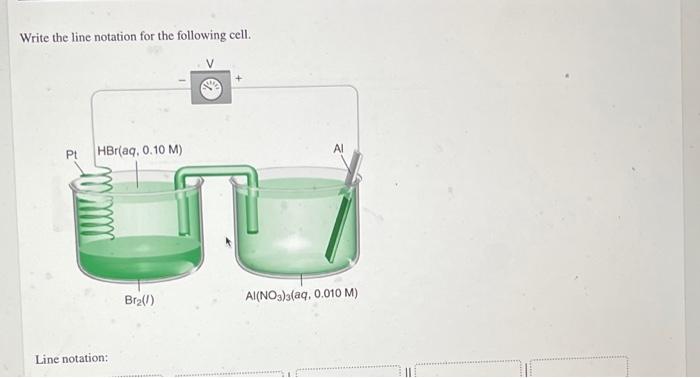
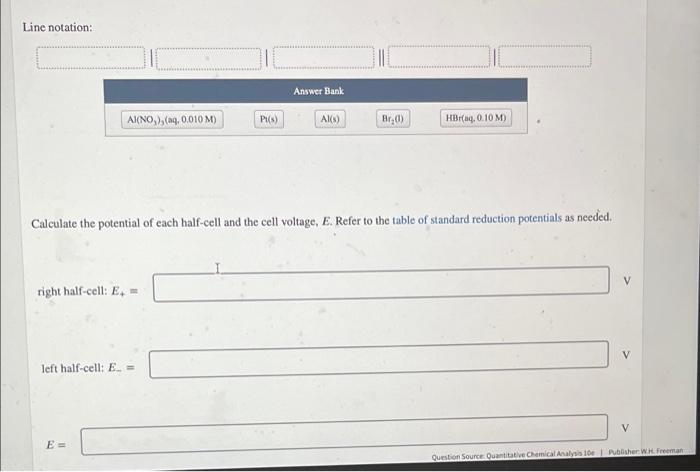
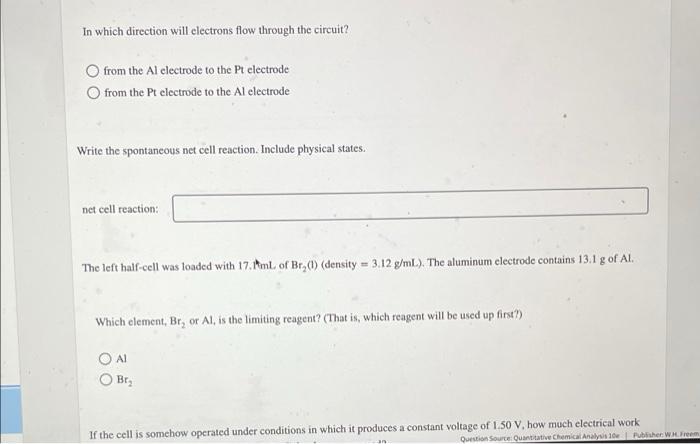

Write the line notation for the following cell. Pt HBr(aq, 0.10 M) Bra() Al(NO3)3(aq, 0.010 M) Line notation: Line notation: Answer Bank AI(NO), (aq, 0.010 M) Pr(s) AK) Br.) HBraq, 0.10 M) Calculate the potential of each half-cell and the cell voltage, E. Refer to the table of standard reduction potentials as needed. V right half-cell: E. left half-cell: E. = V E = Question Source Quantitative Chemical Analysis Publisher W. Freeman In which direction will clectrons flow through the circuit? from the Al electrode to the Pt electrode from the Pt electrode to the Al electrode Write the spontaneous net cell reaction. Include physical states. net cell reaction: The left half-cell was loaded with 17. AmL of Br_() (density = 3.12 g/mL). The aluminum electrode contains 13.1 g of Al. Which element, Br, or Al, is the limiting reagent? (That is, which reagent will be used up first?) AI Bra If the cell is somehow operated under conditions in which it produces a constant voltage of 1.50 V, how much electrical work Question Source Quantitative Chemical Analysis 10 hec Wrem Which element, Br, or Al, is the limiting reagent? (That is, which reagent will be used up first?) Al Br2 If the cell is somehow operated under conditions in which it produces a constant voltage of 1.50 V, how much electrical work will have been done when 0.349 mL of Br, (1) has been consumed? kJ work If the potentiometer is replaced by a 1.45-k2 resistor and if the heat dissipated by the resistor is 1.90 x 10- /s, at what rate (grams per second) is Al(s) dissolving? (In this question, the voltage is not 1.50 V) es rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


