Question: Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of manganese(II) iodide and ammonium carbonate are combined. Be sure to
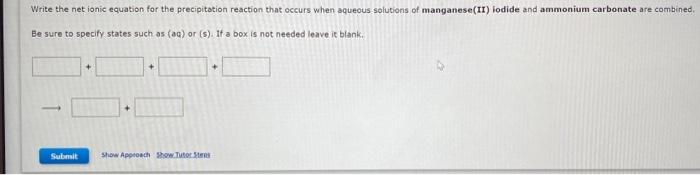
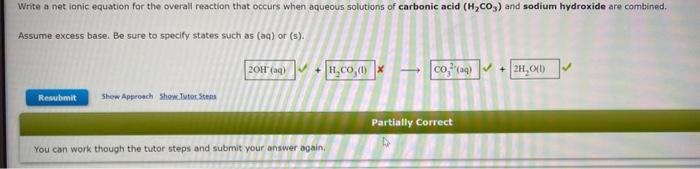
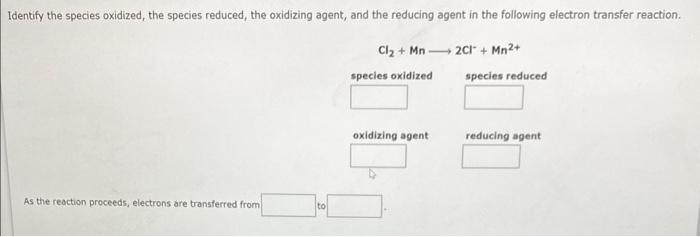
Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of manganese(II) iodide and ammonium carbonate are combined. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.. Write a net ionic equation for the overall reaction that occurs when aqueous solutions of carbonic acid (H2CO3) and sodium hydroxide are combined. Assume excess base. Be sure to specify states such as (aq) or (s). Identify the species oxidized, the species reduced, the oxidizing agent, and the reducing agent in the following electron transfer reaction. Cl2+Mn2Cl+Mn2+ species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


