Question: write uints and correct number of significant figures 2. Answer the following question using the table of initial rate data for the reaction: A+2B2C+D Experiment
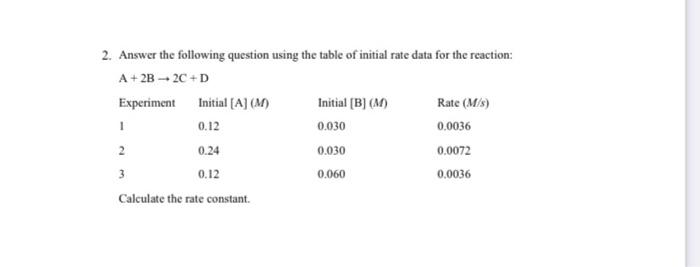
2. Answer the following question using the table of initial rate data for the reaction: A+2B2C+D Experiment Initial [A] (M) Initial [B] (M) Rate (M/s) 0.12 0.0036 2 0.24 0.030 0.0072 3 0.12 0.060 0.0036 Calculate the rate constant. 1 0.030
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


