Question: Write your answer in 4 decimal places, box the final answer and solve neatly. PROBLEM 4 Based from the study of G. N. Lewis and
Write your answer in 4 decimal places, box the final answer and solve neatly.

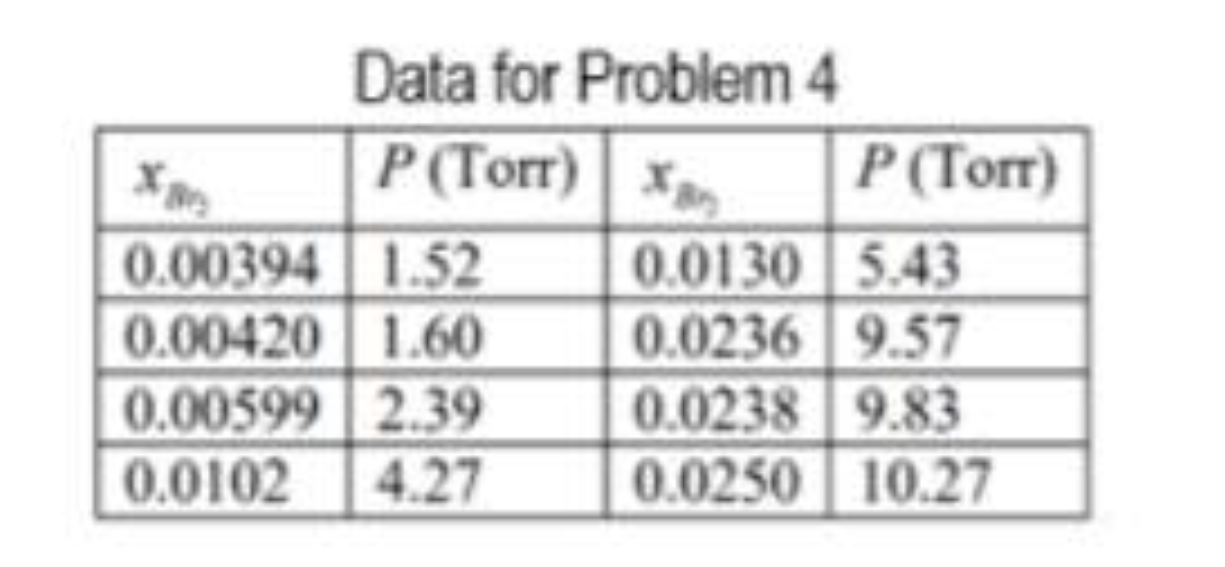
PROBLEM 4 Based from the study of G. N. Lewis and H. Storch, J. American Chemical Society, 1917, the partial pressures of Brz above a solution containing CCIA as the solvent at 25C are found to have the values listed in the table as a function of the mole fraction of Br2 in the solution. Plot the Br2 mole fraction against its partial pressure and from this determine the Henry's gas solubility constant of Br2 in CCl4 at 25C. Hint: The Henry's gas solubility constant is the slope. Data for Problem 4 P (Torr) P (Tor) 0.00394 1.52 0.0130 5.43 0.00420 1.60 0.0236 9.57 0.00599 2.39 0.0238 9.83 0.0102 4.27 0.0250 10.27
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


