Question: - X B. For the reaction N2Os (g) 2 NO2 (g) + O2 (g) AH = 55.2 kJ kis 8.0 x 10-7s-1 at 0.0C and
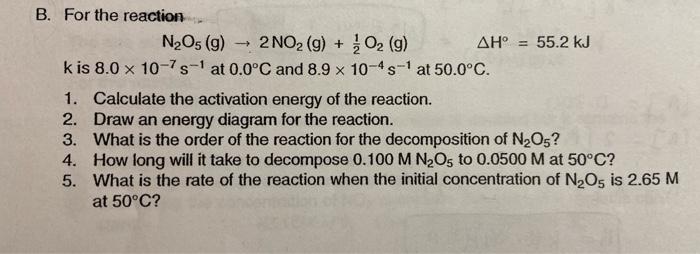
- X B. For the reaction N2Os (g) 2 NO2 (g) + O2 (g) AH = 55.2 kJ kis 8.0 x 10-7s-1 at 0.0C and 8.9 x 10-4s-1 at 50.0C 1. Calculate the activation energy of the reaction. 2. Draw an energy diagram for the reaction. 3. What is the order of the reaction for the decomposition of N2O5? 4. How long will it take to decompose 0.100 M N Os to 0.0500 M at 50C? 5. What is the rate of the reaction when the initial concentration of N2O5 is 2.65 M at 50C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


