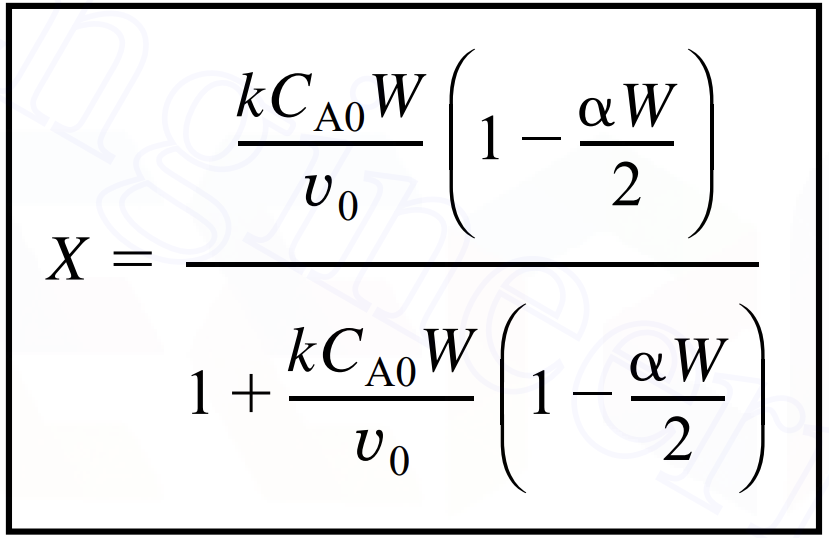
Question: x = k C A 0 W v 0 ( 1 - W 2 ) 1 + k C A 0 W v 0 (
A secondorder reaction is taking place in a packed bed reactor. kg catalyst is loaded in the reactor. For a volumetric flow rate of mh and entering concentration of kmolm find the conversion for kgk mkmolkgcath
bThe company decided to change the catalyst in the reactor. The new catalyst particle diameter is times bigger than the previous one. And also they doubled the entering pressure. If we are in the laminar flow regime, Calculate the new conversion. The reactor is operated at isothermal conditions For the laminar flow regime alpha parameter changes according to the equation below;
A c A c D p D pP P T T
Note: image formula
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


