Question: x please answer all three D Question 7. For the molecular formula CsH100 a. Give the degrees of unsaturation b. Draw four structural isomers that

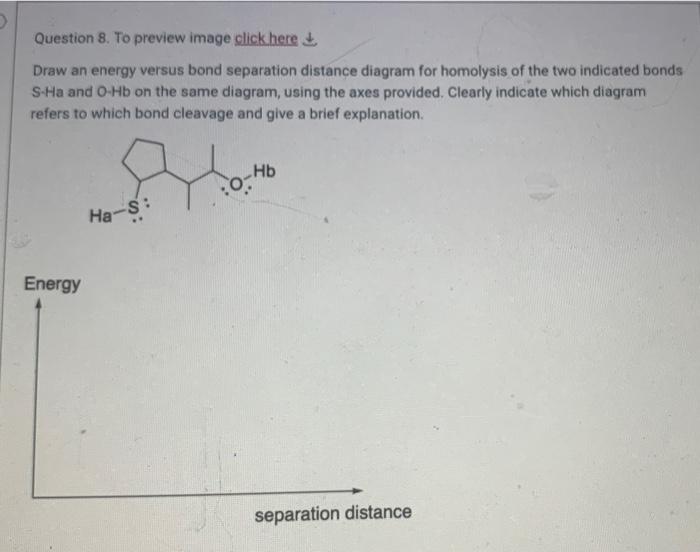
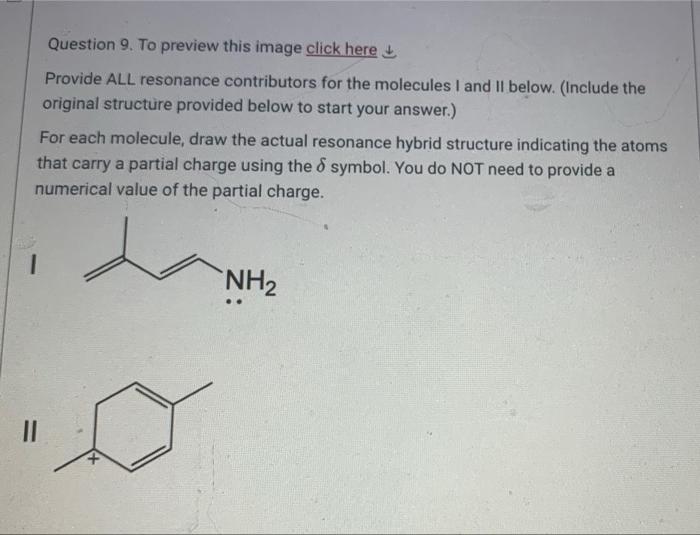
D Question 7. For the molecular formula CsH100 a. Give the degrees of unsaturation b. Draw four structural isomers that obey the normal rules of valence for each atom. Include all non-bonding electrons. You can either draw Lewis structures or line-angle structures. If you draw line angle structures, dont forget to include the H atoms that are normally as part of the functional groups Question 8. To preview image click here Draw an energy versus bond separation distance diagram for homolysis of the two indicated bonds S-Ha and O-Hb on the same diagram, using the axes provided. Clearly indicate which diagram refers to which bond cleavage and give a brief explanation. Hb Ha-S: Energy separation distance Question 9. To preview this image click here Provide ALL resonance contributors for the molecules I and Il below. (Include the original structure provided below to start your answer.) For each molecule, draw the actual resonance hybrid structure indicating the atoms that carry a partial charge using the 8 symbol. You do NOT need to provide a numerical value of the partial charge. NH2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


