Question: X1 Problem 1 0 Using Raoult's Law, predict the system temperature and vapor-phase composition for n-hexane (1)+ethanol (2) system at 1 bar and provide a
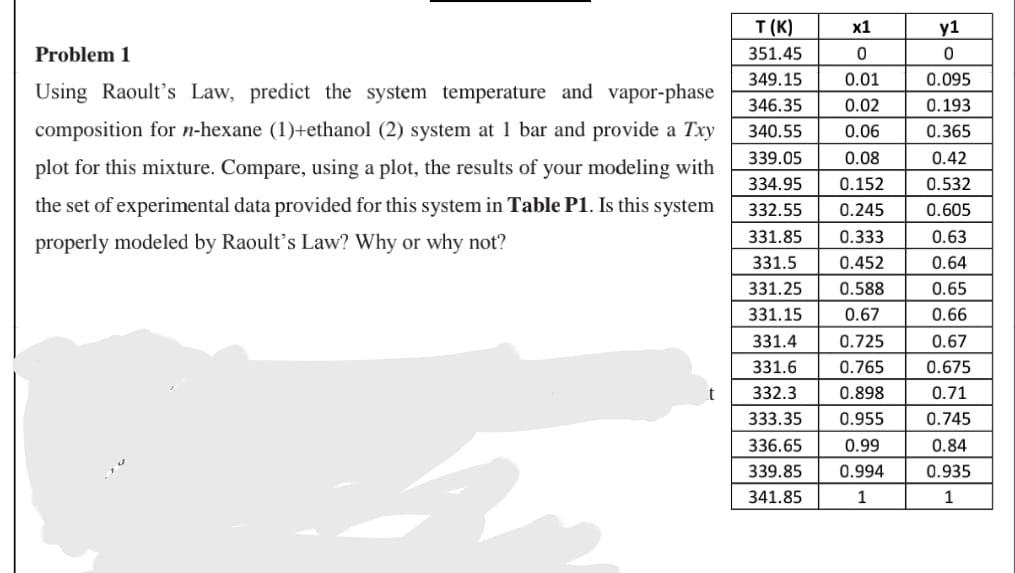
X1 Problem 1 0 Using Raoult's Law, predict the system temperature and vapor-phase composition for n-hexane (1)+ethanol (2) system at 1 bar and provide a Txy plot for this mixture. Compare, using a plot, the results of your modeling with the set of experimental data provided for this system in Table P1. Is this system properly modeled by Raoult's Law? Why or why not? IIIIIIII T(K) 351.45 349.15 346.35 340.55 339.05 334.95 332.55 331.85 331.5 331.25 331.15 331.4 331.6 332.3 333.35 336.65 339.85 341.85 0.01 0.02 0.06 0.08 0.152 0.245 0.333 0.452 0.588 0.67 0.725 0.765 0.898 0.955 0.99 0.994 1 y1 0 0.095 0.193 0.365 0.42 0.532 0.605 0.63 0.64 0.65 0.66 0.67 0.675 0.71 0.745 0.84 0.935 1 t +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


