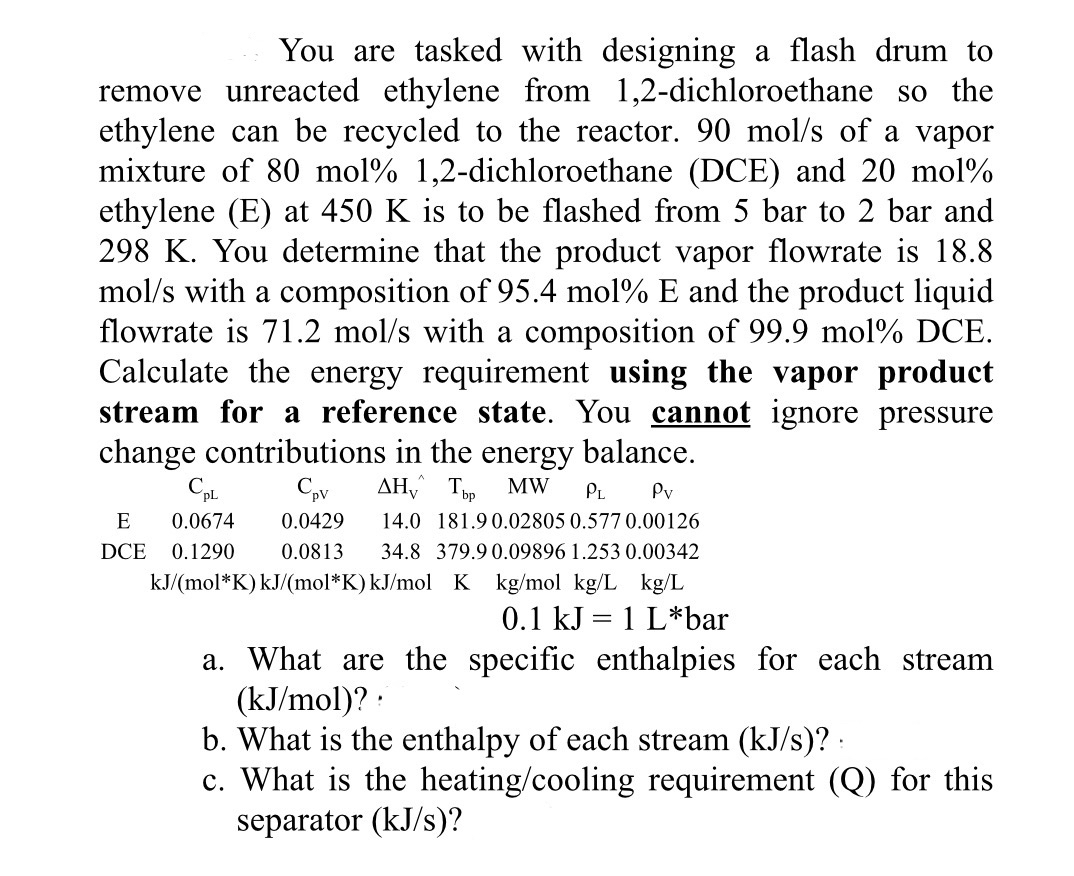
Question: You are tasked with designing a flash drum to remove unreacted ethylene from 1 , 2 - dichloroethane so the ethylene can be recycled to
You are tasked with designing a flash drum to remove unreacted ethylene from dichloroethane so the ethylene can be recycled to the reactor. of a vapor mixture of moldichloroethane DCE and mol ethylene E at is to be flashed from bar to bar and You determine that the product vapor flowrate is with a composition of mol and the product liquid flowrate is with a composition of mol DCE. Calculate the energy requirement using the vapor product stream for a reference state. You cannot ignore pressure change contributions in the energy balance.
a What are the specific enthalpies for each stream :
b What is the enthalpy of each stream
c What is the heatingcooling requirement for this separator
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


