Question: you can plot using excel, but please answer fast and i will rate Question 2 [CO2, PO2, C4) - 10 MARKS A company is having
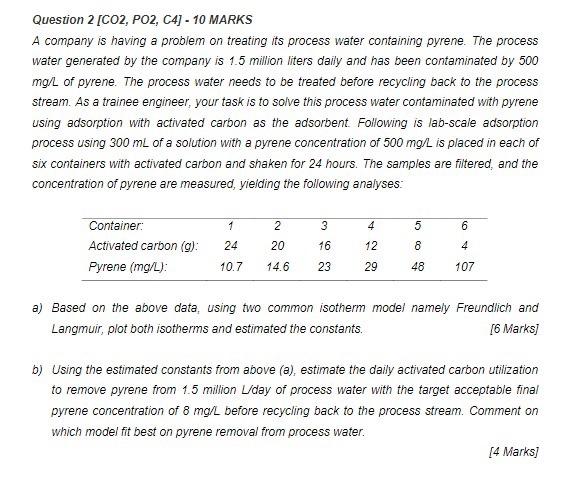
Question 2 [CO2, PO2, C4) - 10 MARKS A company is having a problem on treating its process water containing pyrene. The process water generated by the company is 1.5 million liters daily and has been contaminated by 500 mg/L of pyrene. The process water needs to be treated before recycling back to the process stream. As a trainee engineer, your task is to solve this process water contaminated with pyrene using adsorption with activated carbon as the adsorbent. Following is lab-scale adsorption process using 300 mL of a solution with a pyrene concentration of 500 mg/L is placed in each of six containers with activated carbon and shaken for 24 hours. The samples are filtered and the concentration of pyrene are measured, yielding the following analyses: 1 3 4 6 Container: Activated carbon (g): Pyrene (mg/L): 2 20 5 8 24 16 12 0 4 10.7 14.6 23 29 48 107 a) Based on the above data, using two common isotherm model namely Freundlich and Langmuir, plot both isotherms and estimated the constants. [6 Marks] b) Using the estimated constants from above (e), estimate the daily activated carbon utilization to remove pyrene from 1.5 million U/day of process water with the target acceptable final pyrene concentration of 8 mg/L before recycling back to the process stream. Comment on which model fit best on pyrene removal from process water. [4 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


