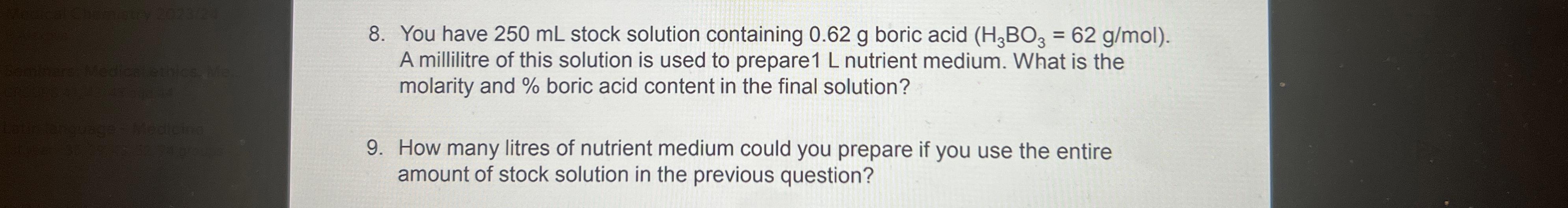
Question: You have 2 5 0 m L stock solution containing 0 . 6 2 g boric acid ) = ( 6 2 g m Mol.
You have stock solution containing boric acid Mol. A millilitre of this solution is used to prepare nutrient medium. What is the molarity and boric acid content in the final solution?
How many litres of nutrient medium could you prepare if you use the entire amount of stock solution in the previous question?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


