Question: Consider the following reaction. What would be the equilibrium constant expression? 4Br2(g) + CH4(9) 4HBr(g) + CBr4(g)
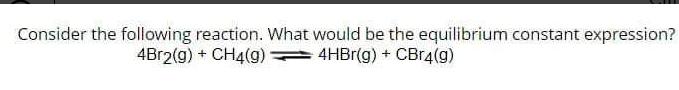
Consider the following reaction. What would be the equilibrium constant expression? 4Br2(g) + CH4(9) 4HBr(g) + CBr4(g)
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Equilibrium constant is the ratio of concentration of products to the react... View full answer

Get step-by-step solutions from verified subject matter experts


