Question: You may use the same answer more than once. Clear All 0.255 moles of Ar in a 5.97 L container at a temperature of 453K
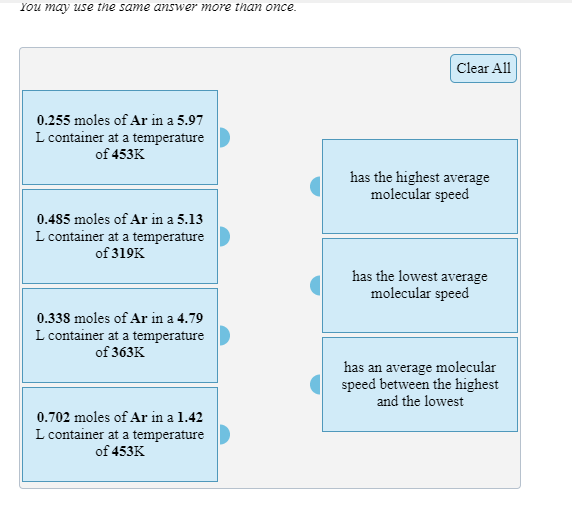
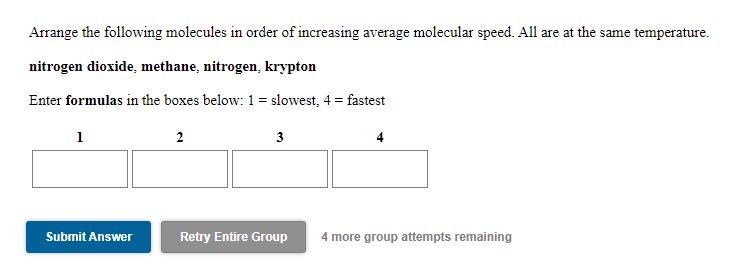
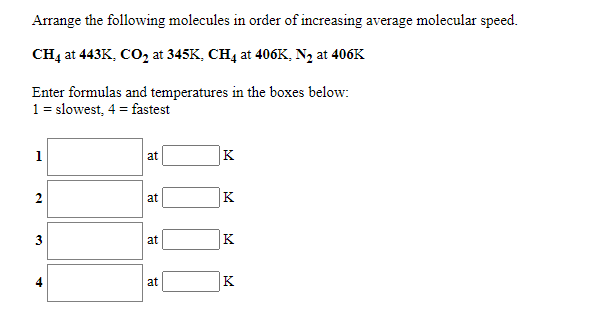
You may use the same answer more than once. Clear All 0.255 moles of Ar in a 5.97 L container at a temperature of 453K has the highest average molecular speed 0.485 moles of Ar in a 5.13 L container at a temperature of 319K has the lowest average molecular speed 0.338 moles of Ar in a 4.79 L container at a temperature of 363K has an average molecular speed between the highest and the lowest 0.702 moles of Ar in a 1.42 L container at a temperature of 453K Arrange the following molecules in order of increasing average molecular speed. All are at the same temperature. nitrogen dioxide, methane, nitrogen, krypton Enter formulas in the boxes below: 1 = slowest, 4 = fastest 1 2 3 4 Submit Answer Retry Entire Group 4 more group attempts remaining Arrange the following molecules in order of increasing average molecular speed. CH4 at 443K, CO2 at 345K, CH4 at 406K, N, at 406K Enter formulas and temperatures in the boxes below: 1 = slowest, 4 = fastest 1 at K 2 at K 3 at K 4 at K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


