Question: You prepare a solution by dissolving 0 . 0 5 0 g of ) = ( 1 1 1 . 1 g m o l
You prepare a solution by dissolving of and of with deionized water into a volumetric flask. Assume these salts dissociate completely and no reactions occur conservative substances calculate
a the ppm concentration of each ionic species
b the concentrations of each ion species
c the molL concentration of each ionic species
d the ionic strength of the solution
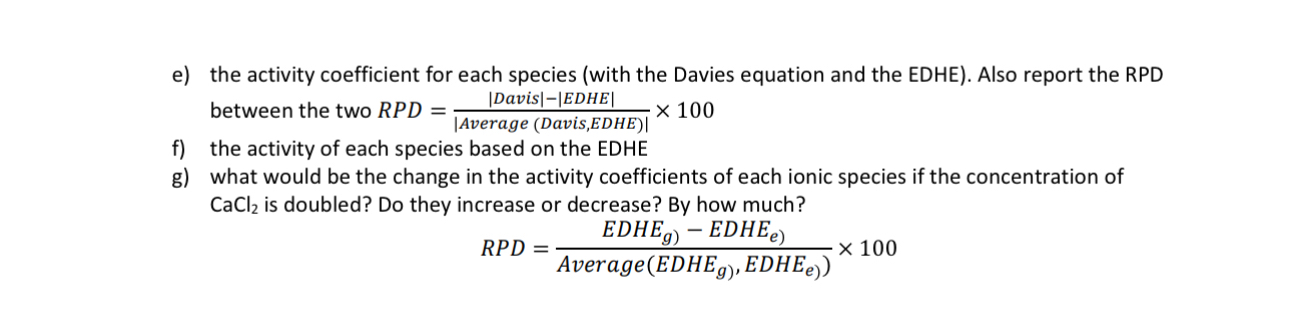
e the activity coefficient for each species with the Davies equation and the EDHE Also report the RPD between the two
f the activity of each species based on the EDHE
g what would be the change in the activity coefficients of each ionic species if the concentration of is doubled? Do they increase or decrease? By how much?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


