Question: You will use the Gas Properties simulation for this lab. Start in the Ideal tab. Note: PV=NRT 1. Check width on the right so
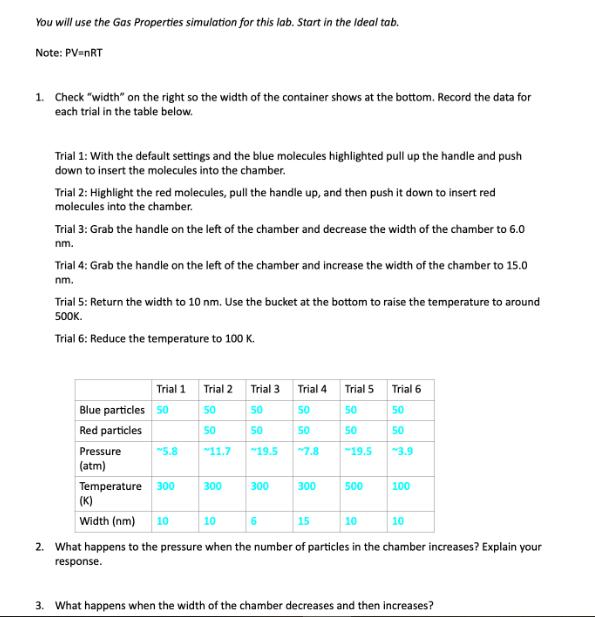

You will use the Gas Properties simulation for this lab. Start in the Ideal tab. Note: PV=NRT 1. Check "width" on the right so the width of the container shows at the bottom. Record the data for each trial in the table below. Trial 1: With the default settings and the blue molecules highlighted pull up the handle and push down to insert the molecules into the chamber. Trial 2: Highlight the red molecules, pull the handle up, and then push it down to insert red molecules into the chamber. Trial 3: Grab the handle on the left of the chamber and decrease the width of the chamber to 6.0 nm. Trial 4: Grab the handle on the left of the chamber and increase the width of the chamber to 15.0 nm. Trial 5: Return the width to 10 nm. Use the bucket at the bottom to raise the temperature to around 500K. Trial 6: Reduce the temperature to 100 K. Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Trial 6 Blue particles 50 50 50 50 50 50 Red particles 50 50 50 50 50 Pressure 5.8 11.7 19.5 7.8 19.5 3.9 (atm) Temperature 300 300 300 300 500 100 (K) Width (nm) 10 10 15 10 10 2. What happens to the pressure when the number of particles in the chamber increases? Explain your response. 3. What happens when the width of the chamber decreases and then increases? 4. What happens when the temperature is raised and then decreased? Explain. 5. Choose something to hold constant on the top right and something to change. State the variable being held constant and the one being changed below, then predict the effect on the chamber with an explanation of why you predict that effect. 6. Test your prediction from question 6 and report your results. 7. Why is the ideal lab not a good predictor of what will happen in real life? What can it tell us that is beneficial?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


