Question: YY eral Posts Files Class Notebook Fill | chem137 Final ex... 6 E 6 more 2. Calculate the standard free energy change for the following
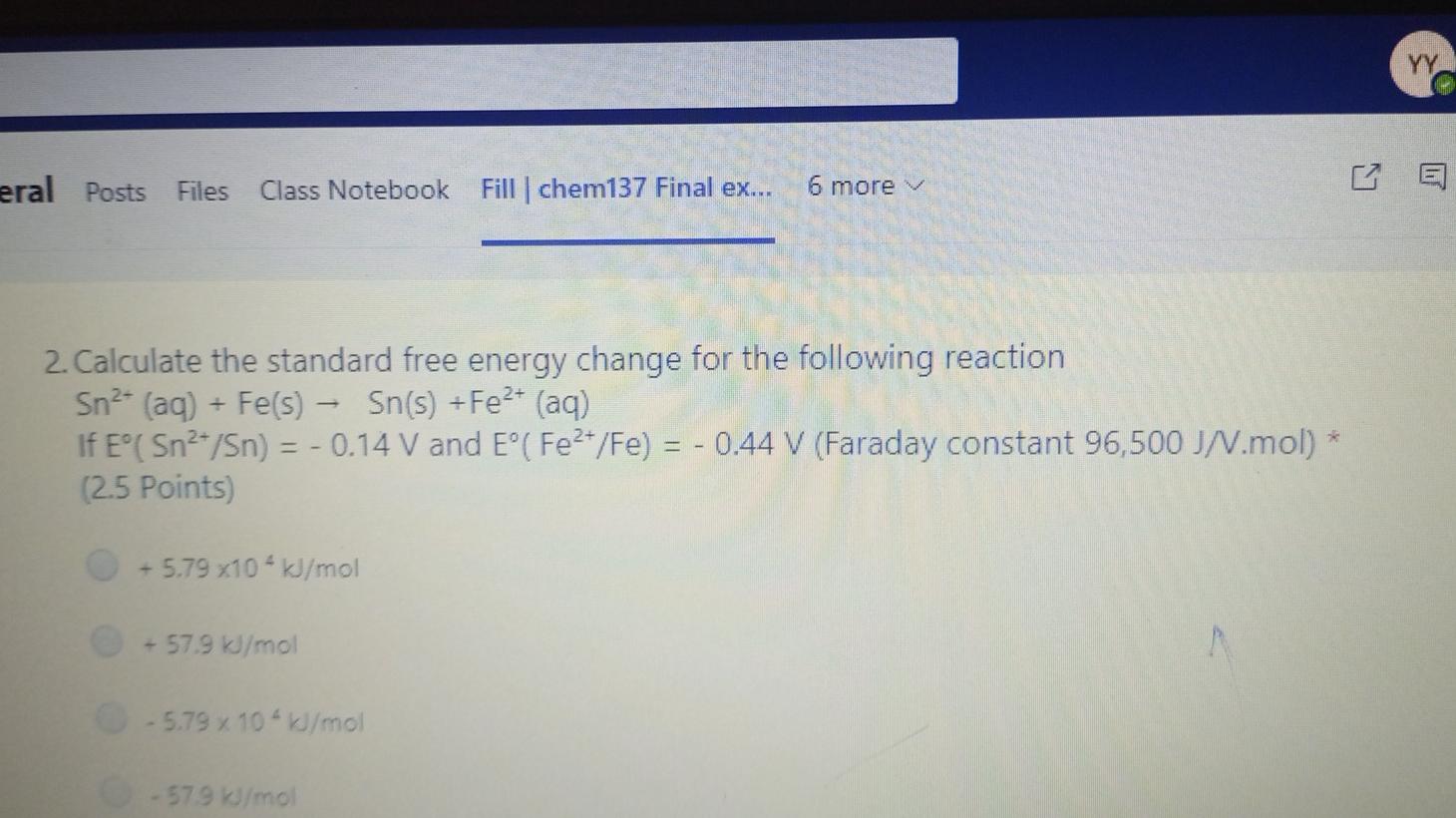
YY eral Posts Files Class Notebook Fill | chem137 Final ex... 6 E 6 more 2. Calculate the standard free energy change for the following reaction Sn?- (aq) + Fe(s) - Sn(s) +Fe2+ (aq) If E(Sn2/Sn) = -0.14 V and E( Fe2+/Fe) = -0.44 V (Faraday constant 96,500 J/V.mol) * (2.5 Points) +5.79 x104 kJ/mol + 57.9 kJ/mol - 5.79 x 104 kJ/mol 579 kJ/mol YY eral Posts Files Class Notebook Fill | chem137 Final ex... 6 E 6 more 2. Calculate the standard free energy change for the following reaction Sn?- (aq) + Fe(s) - Sn(s) +Fe2+ (aq) If E(Sn2/Sn) = -0.14 V and E( Fe2+/Fe) = -0.44 V (Faraday constant 96,500 J/V.mol) * (2.5 Points) +5.79 x104 kJ/mol + 57.9 kJ/mol - 5.79 x 104 kJ/mol 579 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


