An acetoneethanol mixture of 0.5 mole fraction acetone is to be separated by batch distillation at 101
Question:
An acetone–ethanol mixture of 0.5 mole fraction acetone is to be separated by batch distillation at 101 kPa.
Vapor–liquid equilibrium data at 101 kPa are as follows:
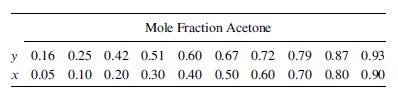
(a) Assuming an L=D of 1.5 times the minimum, how many stages should this column have if the desired composition of the distillate is 0.90 mole fraction acetone when the residue contains 0.1 mole fraction acetone?
(b) Assume the column has eight stages and the reflux rate is varied continuously so that the top product is maintained constant at 0.9 mole fraction acetone. Make a plot of the reflux ratio versus the still-pot composition and the amount of liquid left in the still-pot.
(c) Assume the same distillation is carried out at constant reflux ratio (and varying product composition). It is desired to have a residue containing 0.1 and an (average) product containing 0.9 mole fraction acetone. Calculate the total vapor generated. Which method of operation is more energy-intensive? Suggest operating policies other than constant reflux ratio and constant distillate compositions that lead to equipment or operating cost savings.
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





