For ethanol(1)-isooctane(2) mixtures at 50 C, the infinitedilution, liquid-phase activity coefficients are and (a) Calculate the
Question:
For ethanol(1)-isooctane(2) mixtures at 50οC, the infinitedilution, liquid-phase activity coefficients are ![]() and
and ![]()
(a) Calculate the cnstants A12 and A21 in the van Laar equations.
(b) Calculate the constants L12 and L21 in the Wilson equations.
(c) Using the constants from (a) And (b), Calculate Υ1 and Υ2 over the composition range and plot the points as log g versus x1.
(d) How well do the van Laar and Wilson predictions agree with the azeotropic point x1 = 0.5941, Υ1 1.44, and Υ2 = 2.18?
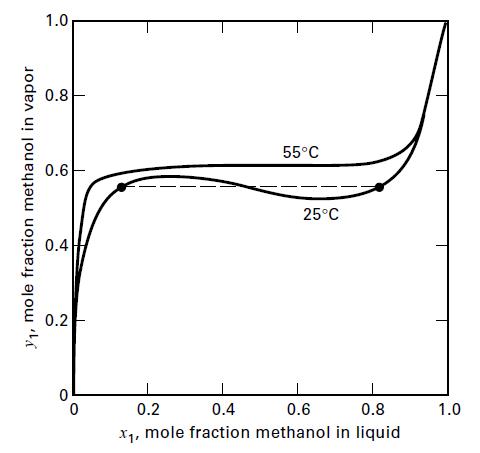
(e) Show that the van Laar equation erroneously predicts two liquid phases over a portion of the composition range by calculating and plotting a y–x diagram like Figure 2.16.
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





